(C) 2012 Michael G. Rix. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Assassin Spiders of the family Archaeidae from southern Australia are revised, with a new genus (Zephyrarchaea gen. n.) and nine new species described from temperate, mesic habitats in southern Victoria, South Australia and south-western Western Australia: Zephyrarchaea austini sp. n., Zephyrarchaea barrettae sp. n., Zephyrarchaea grayi sp. n., Zephyrarchaea janineae sp. n., Zephyrarchaea marae sp. n., Zephyrarchaea marki sp. n., Zephyrarchaea melindae sp. n., Zephyrarchaea porchi sp. n. and Zephyrarchaea vichickmani sp. n. Specimens of the type species, Zephyrarchaea mainae (Platnick, 1991), comb. n., are redescribed from the Albany region of Western Australia, along with the holotype female of Zephyrarchaea robinsi (Harvey, 2002) comb. n. from the Stirling Range National Park. The previously described species Archaea hickmani Butler, 1929 from Victoria is here recognised as a nomen dubium. A key to species and multi-locus molecular phylogeny complement the species-level taxonomy, with maps, habitat photos, natural history information and conservation assessments provided for all species.
New species, taxonomy, conservation, cytochrome c oxidase , mitochondrial DNA, Palpimanoidea
Once considered among the most enigmatic and poorly known of spider families, recent research into the assassin spiders of the family Archaeidae (Fig. 1) has revealed three diverse, highly endemic faunas from southern Africa, Madagascar and Australia, each of considerable evolutionary and conservation significance, and all the focus of modern revisionary systematic studies that have transformed our understanding of archaeid evolution and biogeography (see Platnick 1991a, 1991b, Lotz 1996, 2003, 2006, Harvey 2002a, Wood et al. 2007, Wood 2008, Rix and Harvey 2011, 2012). Although widely known from Mesozoic and Tertiary fossil deposits on multiple continents (see Dunlop et al. 2012), the Recent archaeid fauna consists of 54 described species in three genera (Platnick 2012), with numerous new species still to be described from African, Malagasy and Australian regions (H. Wood, pers. comm., M. Rix pers. obs.). These species, all remarkable for their araneophagic ecology and highly autapomorphic morphology, have highlighted the contrasting patterns of speciation and endemism that occur in Australian versus Old World taxa, and the importance of the unique archaeid carapace morphology in the evolution of the group (see Wood et al. 2007, Rix and Harvey 2012). The distinctive Australian fauna, while long neglected taxonomically and once presumed to be comparatively species-poor, has recently been shown to be far more widespread and species-rich than previously expected, thanks to dedicated field surveys and significant advances in our understanding of archaeid biology, ecology and biogeography (see Rix and Harvey 2011, 2012).
The history of the discovery and documentation of Archaeidae in Australia is one of significant recent, almost exponential progress, with over 85% of currently recognised species described in the past five years, and only four valid taxa described in the eight decades since the first species, Austrarchaea hickmani (Butler, 1929) was first recorded from Victoria. The second true archaeid to be recorded from Australia, Austrarchaea nodosa (Forster, 1956), was described from the Lamington Plateau, south-eastern Queensland, and nearly 30 years later Forster and Platnick (1984) described a third species, Austrarchaea daviesae Forster & Platnick, 1984, from the Wet Tropics of north-eastern Queensland, further erecting the new genus Austrarchaea to include all of the Australian taxa. Archaeidae were unknown from Western Australia until Austrarchaea mainae Platnick, 1991b (Figs 1E-F) was described from the Torndirrup Peninsula, near Albany (first reported by Dyer and Lyon 1983, Main 1987a, 1987b), and Main (1995) reported the discovery in the 1970s of six unidentified juvenile specimens from near Pemberton. The first archaeid specimen to be recorded from the Stirling Range National Park in southern Western Australia was collected in 1996 and this species, Austrarchaea robinsi Harvey, 2002a, remained the only assassin spider to have been collected in Western Australia for a further 10 years. In 2006, living adult specimens of Zephyrarchaea janineae sp. n. (Fig. 1C) were discovered in wet forest near Pemberton, catalysing the first of many such discoveries in Western Australia, and highlighting the importance of temperate coastal heathlands as a habitat for assassin spiders. Indeed, since the pioneering revisionary work of Forster and Platnick (1984), when only eight adult archaeids had been recorded for the whole of Australia, over 500 specimens of Archaeidae have since been found throughout Queensland, New South Wales, Victoria, South Australia and Western Australia, revealing a diverse Australian fauna, characterised by numerous new species and mostly allopatric, relictual, short-range endemic taxa (Harvey 2002b, Harvey et al. 2011, Rix and Harvey 2011, 2012).
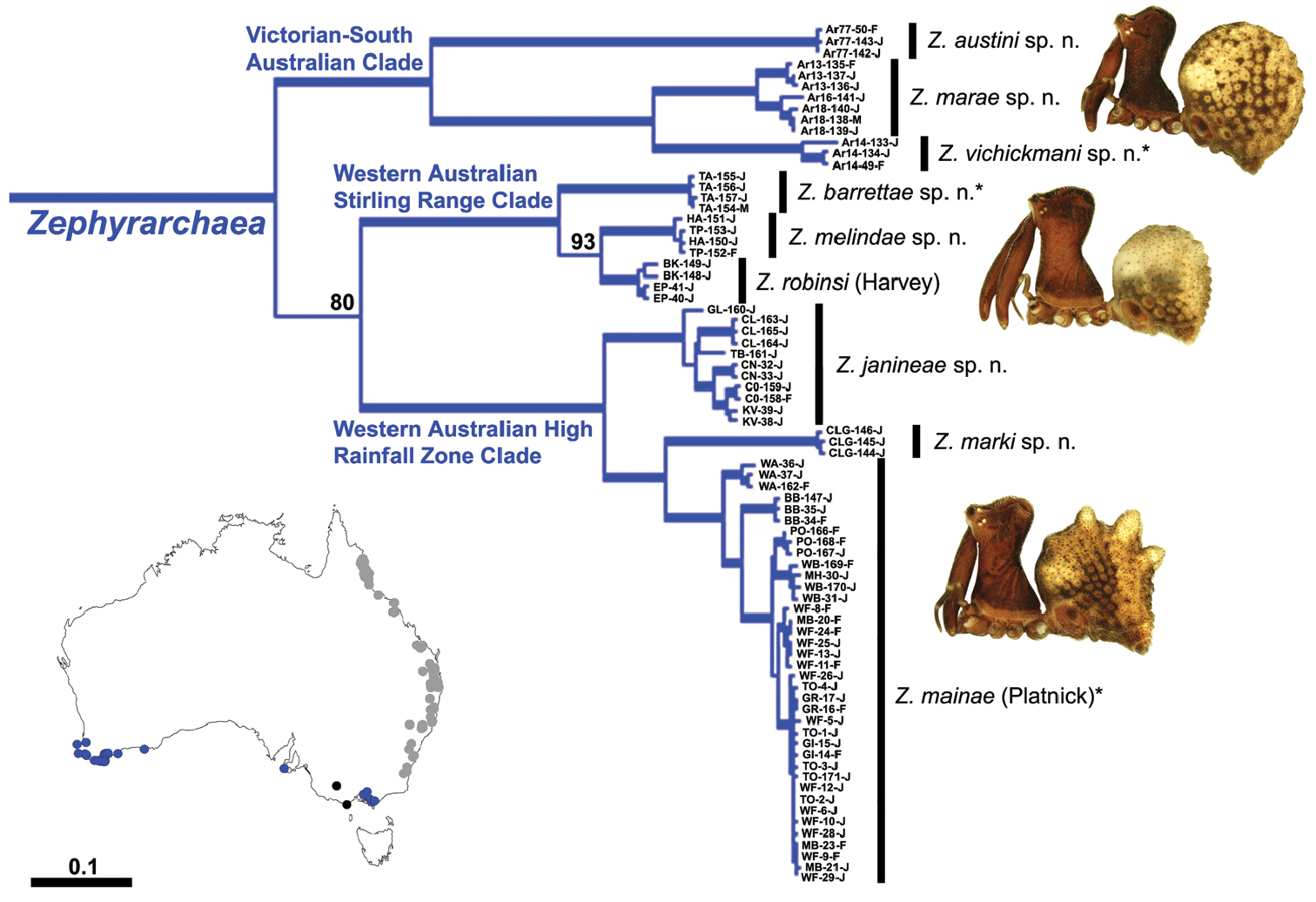
The current paper – the second in a series revising the Archaeidae of Australia – presents a taxonomic revision of the assassin spiders from temperate ‘southern Australia’, including those species from Victoria, South Australia and south-western Western Australia (Fig. 2). A new genus, Zephyrarchaea (Figs 1, 4), is described to include the type species, Zephyrarchaea mainae, along with Zephyrarchaea robinsi and nine new species. These taxa were found to form a monophyletic and highly divergent clade in a recent molecular phylogenetic analysis (Rix and Harvey 2012; Fig. 3), sister to all other species of Archaeidae from eastern Australia. This revision takes the total number of described Australian Archaeidae to 30 species, with the remaining, unrevised archaeids from north-eastern Queensland to be described in the third and final monograph of this series.
Material and methodsAll taxa were described and illustrated from specimens stored in 75% or 95% ethanol. Digital images were taken using a Leica MZ16A binocular microscope and a Leica DM2500 compound microscope, with auto-montage images captured using Leica DFC500 mounted cameras with Leica Application Suite Version 3.6.0 software. Male left pedipalps were dissected prior to imaging and bulbs were aligned for standardised comparison in the retrolateral and prolateral positions illustrated; expanded pedipalps were illustrated in a retro-ventral position. Female genitalia were dissected and cleared in a 10% lactic acid plus 90% glycerol solution, prior to mounting on temporary glass slides. Illustrations were made on Utoplex tracing paper, using printed template auto-montage images. Maps were generated using ArcMap version 9.3.1 (ESRI Inc.) with Virtual Earth (Microsoft Corp.).
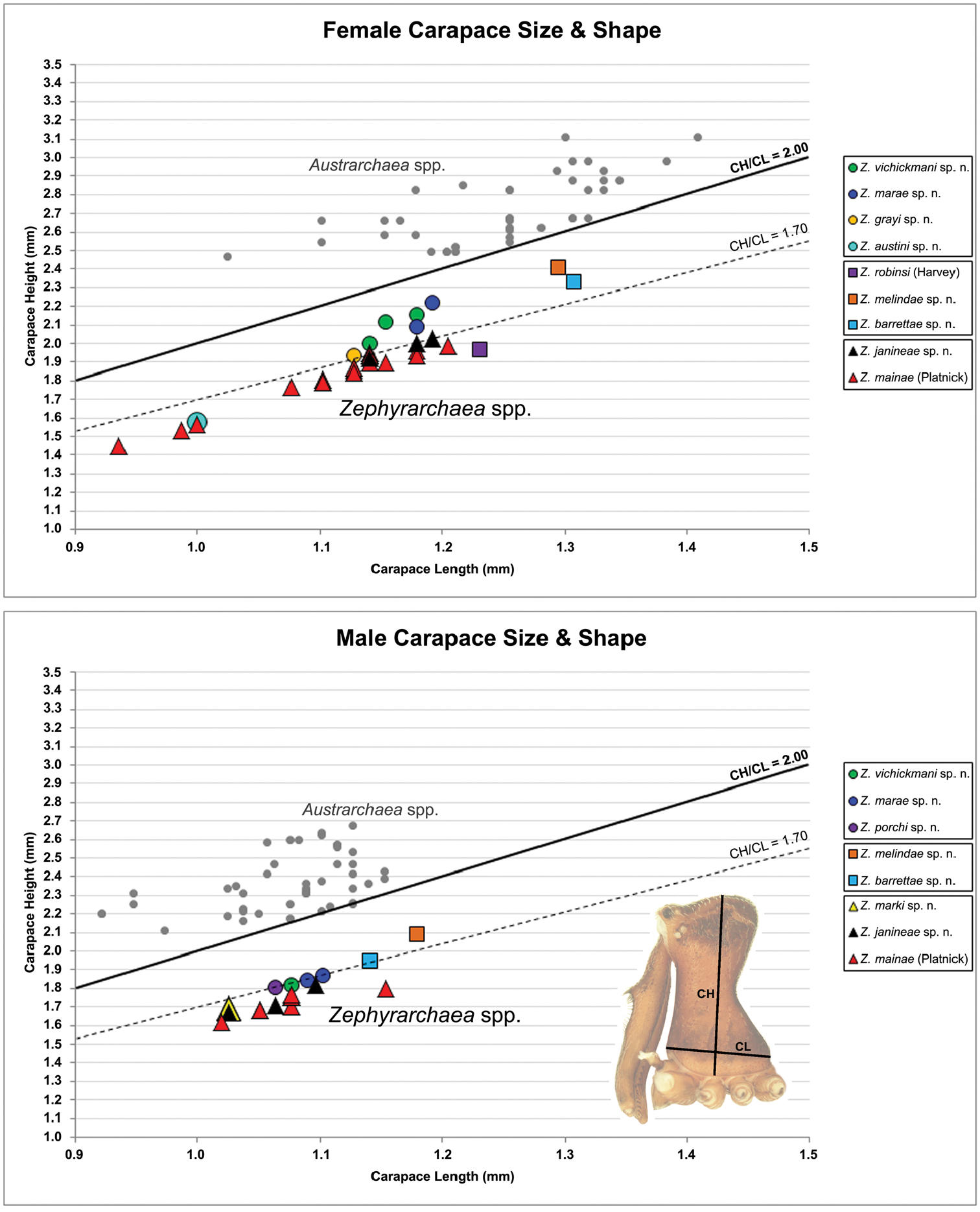
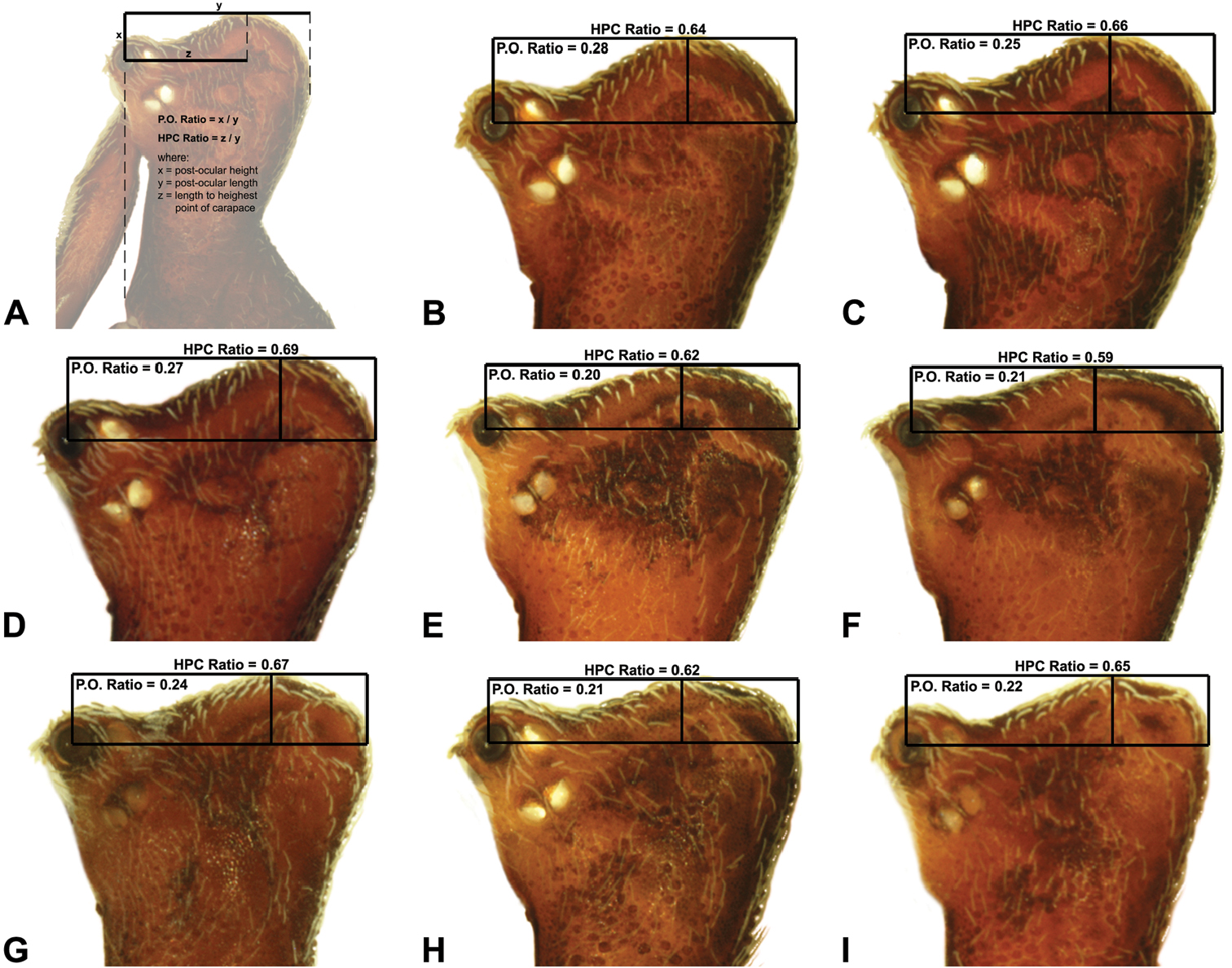
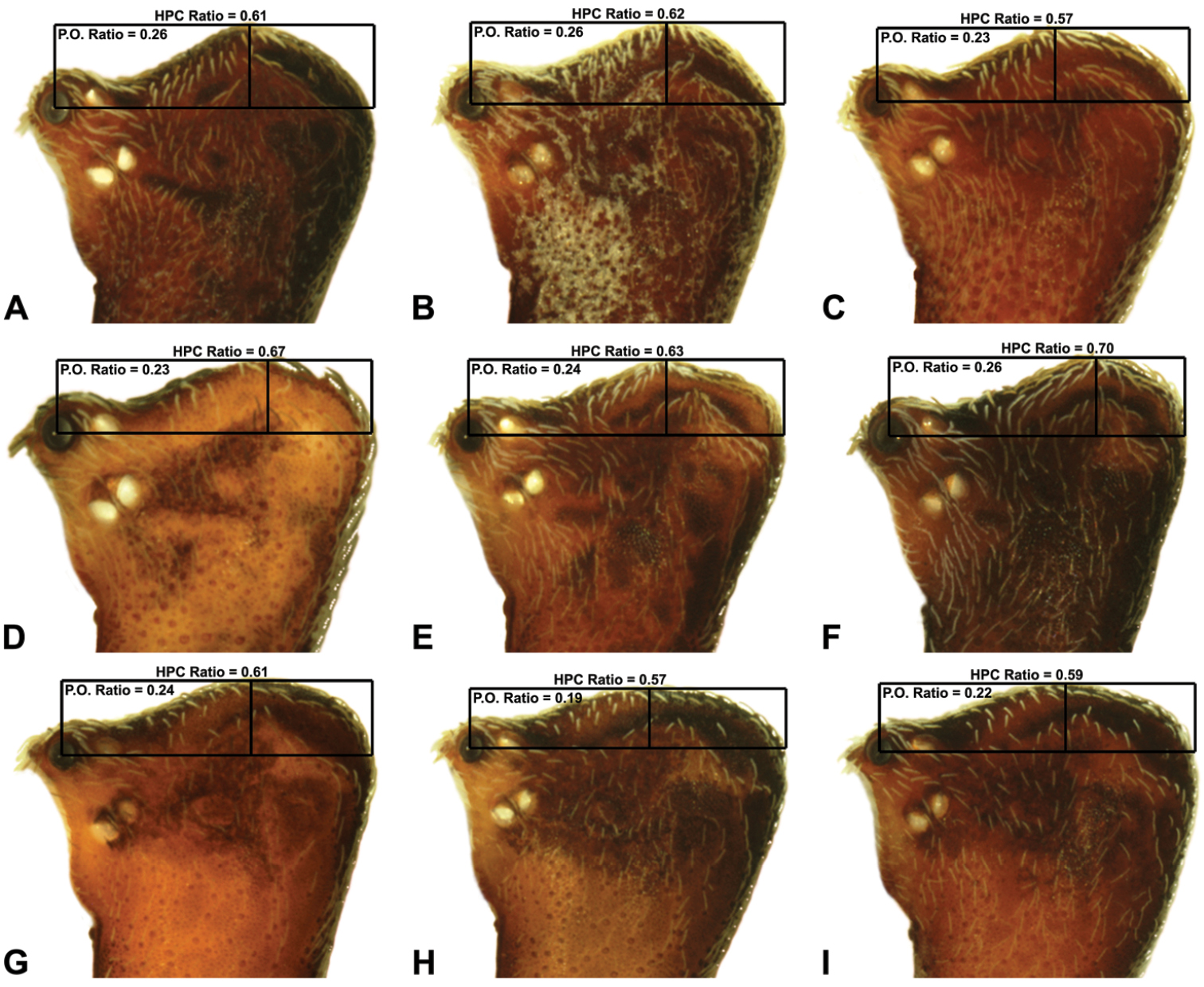
Measurements are in millimetres (rounded to the nearest hundredth of a millimetre) and were taken using an ocular graticule on a Leica M80 binocular microscope. Left legs were removed from specimens prior to taking measurements and imaging lateral body profiles. Lateral profile images were standardised for inter-specific comparison by vertically aligning the centre of each left anterior median eye with the lower anterior margin of the carapace (above the labrum) (Fig. 8A). Carapace height was measured in lateral view, from the margin of the pars thoracica above coxa II to the highest point of the pars cephalica (Fig. 7). Carapace length was measured from the lower anterior margin of the carapace (above the labrum) to the posterior margin of the pars thoracica (above the pedicel) (Fig. 7). ‘Neck’ width was measured in lateral view, at the narrowest point of the carapace, with total length, carapace width, abdomen length and abdomen width all measured in dorsal view. To quantify inter-specific variation in the shape of the cephalothorax and ‘head’, three morphometric ratios (the carapace height to carapace length [CH/CL] ratio; the post-ocular ratio [P.O. ratio]; and the ratio of highest point of pars cephalica [HPC] to post-ocular length ratio) were derived from lateral profile images (Figs 7-9) as defined and discussed by Rix and Harvey (2011) (see also Fig. 8A).
ConventionsSpecimens sequenced for the molecular analysis of Rix and Harvey (2012) are denoted by superscript codes, which correspond to specimen codes as shown in Rix and Harvey (2012, table 1, fig. 4). For species diagnoses, molecular autapomorphies for mitochondrial cytochrome oxidase genes (COI-COII; see Harvey et al. 2008, Cook et al. 2010, Rix and Harvey 2011) are coded according their nucleotide number (1-1609), as defined in Rix and Harvey (2011, table 3).
Abbreviations used in the text are as follows:AME Anterior median eye/s
C1-2 Conductor sclerites 1–2
CH/CL Carapace height (CH) to carapace length (CL) ratio
F1/CL Femur I length (F1) to carapace length (CL) ratio
HPC Highest point of pars cephalica
HT 1–6 Abdominal hump-like tubercles 1–6
PME Posterior median eye/s
TS 1–3 Tegular sclerites 1–3
Specimens described in this study are lodged at the following institutions:AMNH American Museum of Natural History, New York (N. Platnick, L. Sorkin)
AMS Australian Museum, Sydney (G. Milledge)
CASENT California Academy of Sciences, San Francisco (C. Griswold, A. Carmichael)
MV Museum Victoria, Melbourne (P. Lillywhite)
QMB Queensland Museum, Brisbane (R. Raven, O. Seeman)
SAM South Australian Museum, Adelaide (L. Chisholm)
WAM Western Australian Museum, Perth (MSH, J. Waldock)
Taxonomy Family Archaeidae Koch & Berendt, 1854urn:lsid:zoobank.org:act:BEE3BD64-0A61-40D4-880A-2A562847A855
Austrarchaea mainae Platnick, 1991b.
The generic name is derived from the Latin ‘zephyrus’, meaning ‘west wind’ (Brown 1956), in reference to the diversity of this genus in south-western Australia, and the windy, coastal habitats occupied by several species, including the type species.
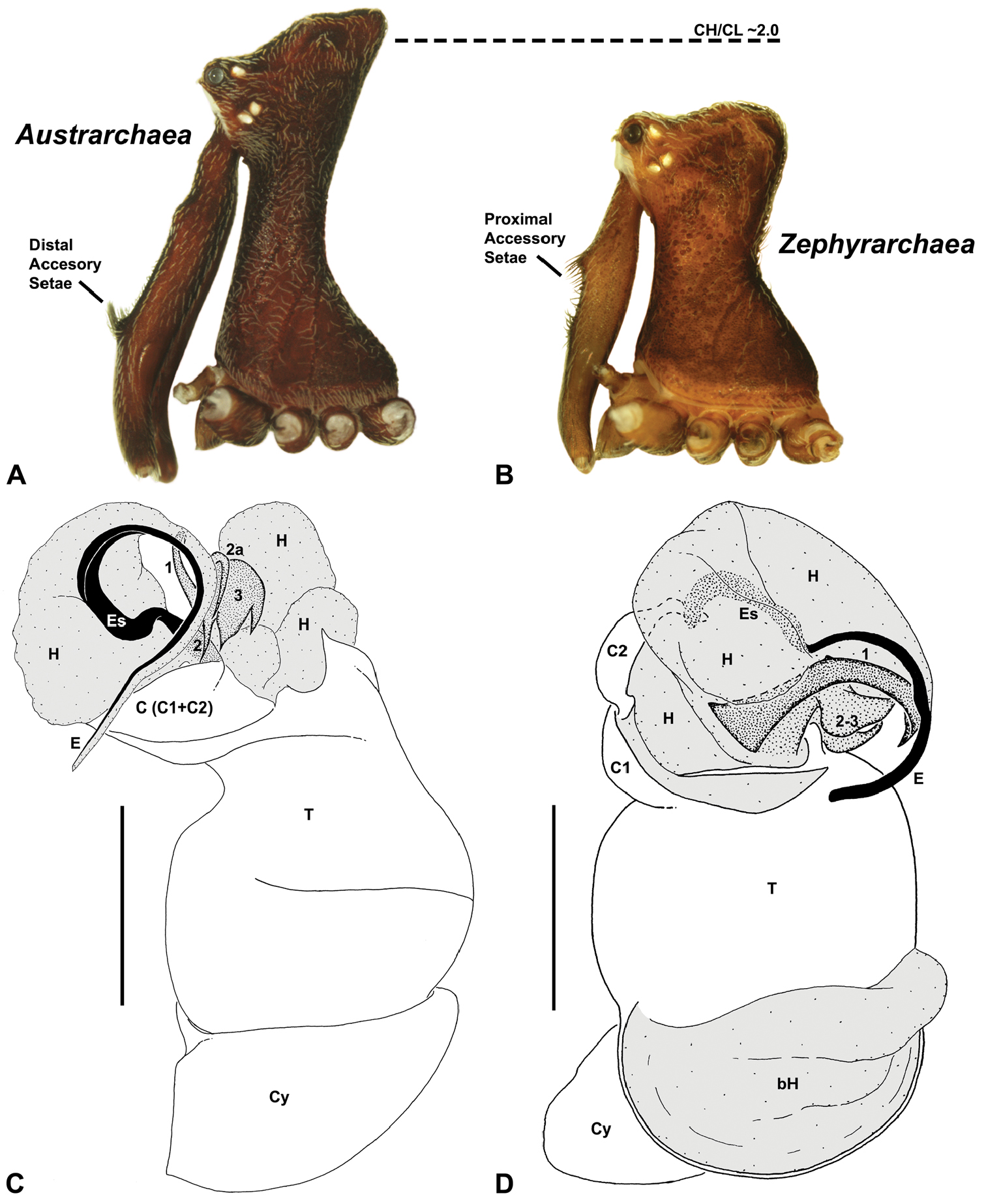
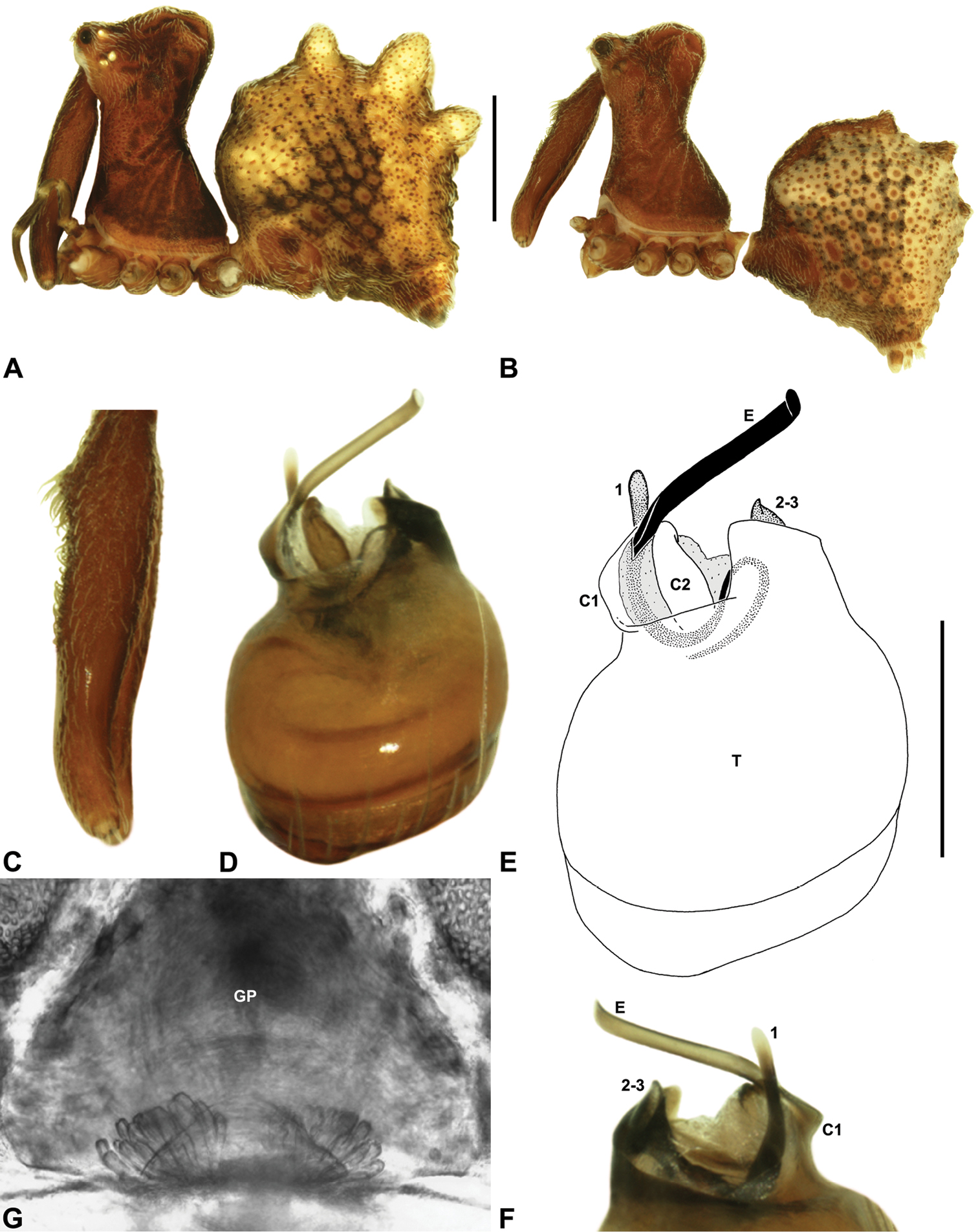
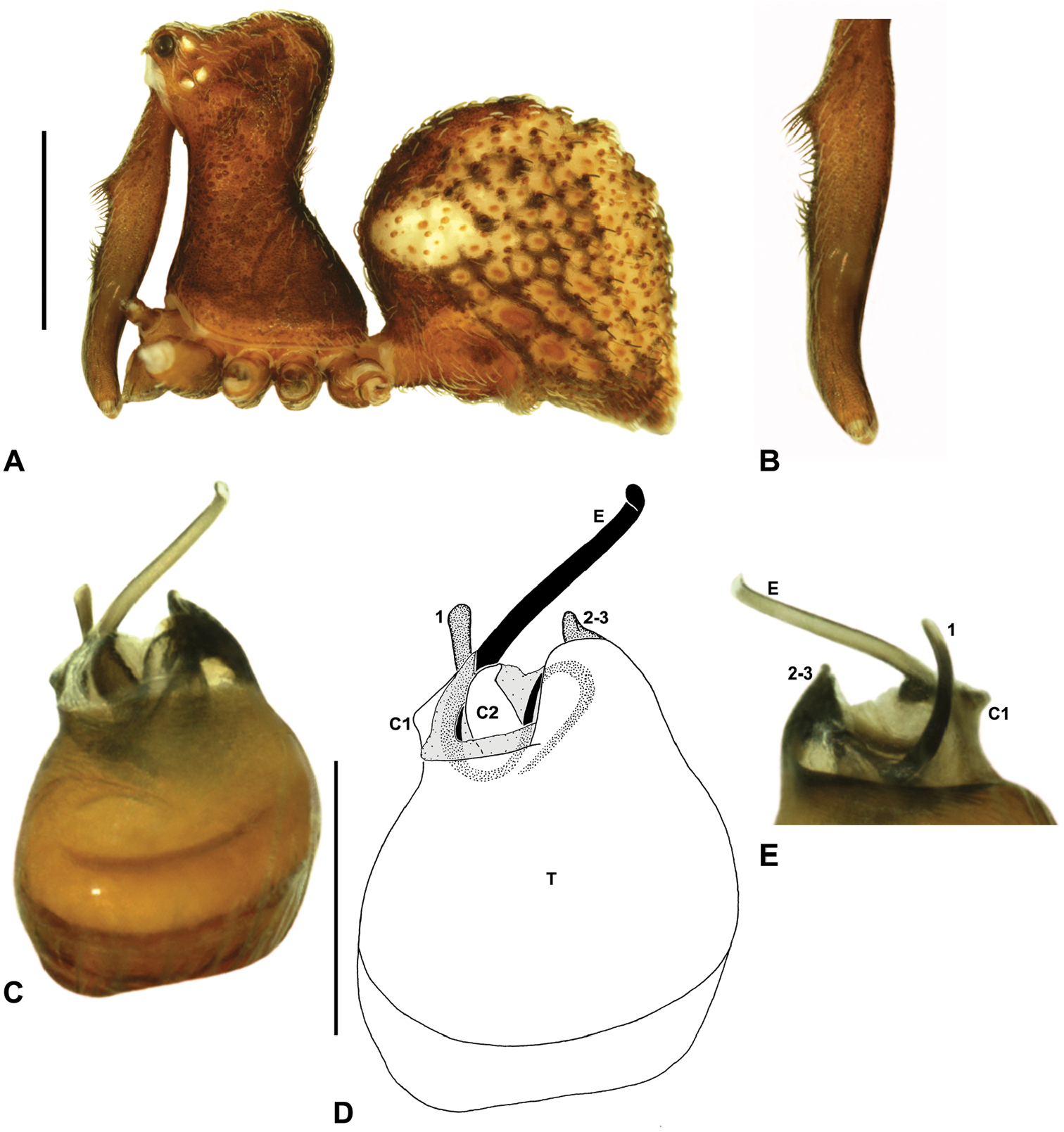
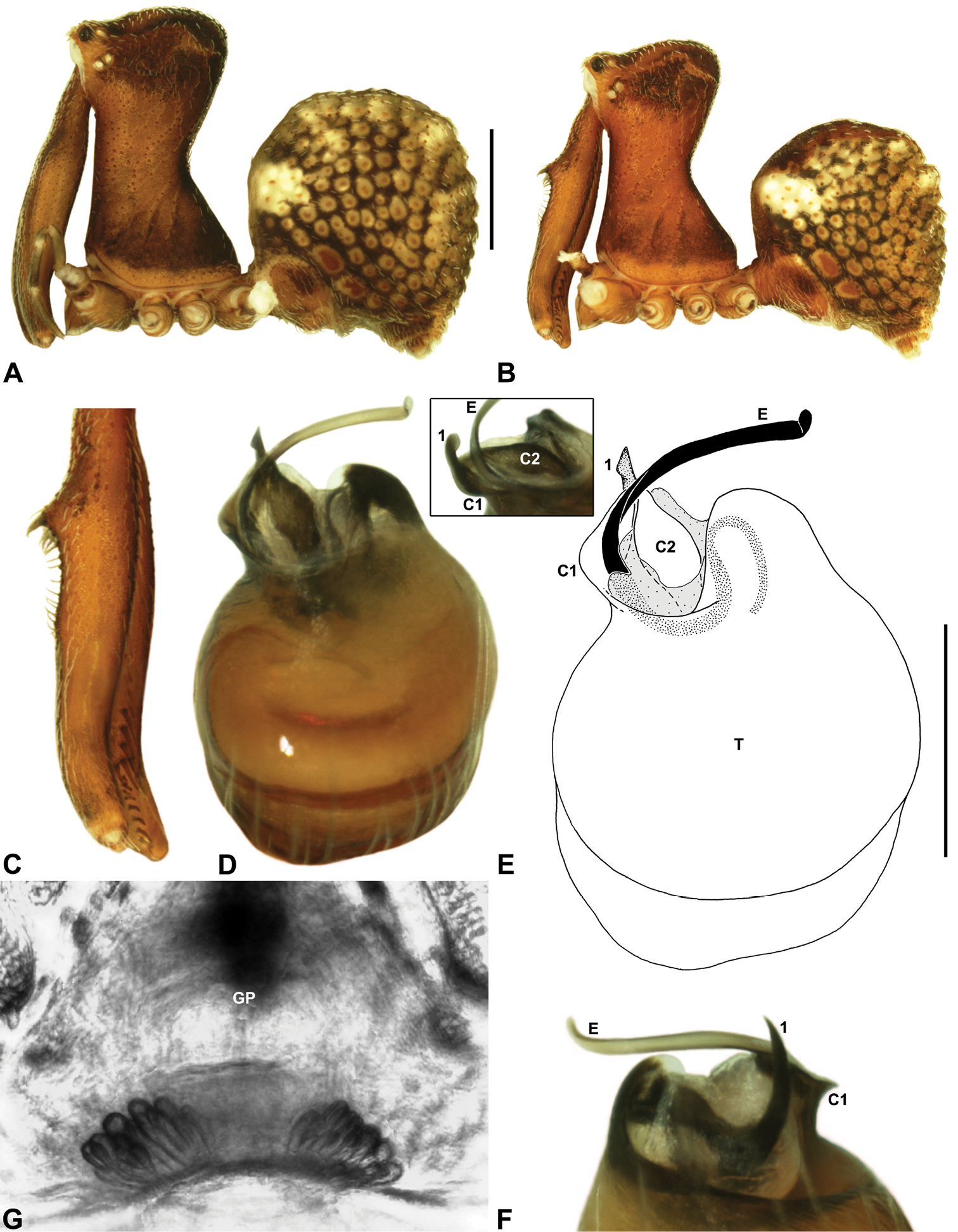
Species of Zephyrarchaea can be distinguished from all eastern Australian species of Austrarchaea by the significantly shorter carapace (CH/CL ratio << 2.0) (Fig. 4B cf. Fig. 4A, 7), by the presence of accessory setae on or adjacent to the proximal (rather than the distal) bulge of the male cheliceral paturon (Fig. 4B cf. Fig. 4A), and by the shape of the two conductor sclerites on the male pedipalp, which are hinged, unfused and moveable (Fig. 4D cf. Fig. 4C), together forming a fully articulated cradle for the unexpanded embolus (Fig. 10E). Like species of Austrarchaea, the genus can be further distinguished from Malagasy and African species of Eriauchenius and Afrarchaea by the presence of numerous, clustered spermathecae in females (Fig. 16G) and by the presence of a long, wiry embolus on the pedipalp of males (Figs 10E, 16E) (Forster and Platnick 1984, Wood 2008, Rix and Harvey 2011).
Small, haplogyne, araneomorph spiders; total length 2.5 to 4.5.
Colouration: Body colouration cryptic and relatively uniform across species, usually with only subtle intraspecific variation in abdominal patterning; carapace, sternum and chelicerae tan brown to reddish-brown in males, interspersed with darker regions of granulate cuticle (Figs 5F–G), covered in highly reflective setae; legs tan-brown to darker reddish-brown, with pattern of darker annulations on distal segments; abdomen mottled with beige and variable hues of grey-brown (Fig. 6), with reddish-brown sclerites, scutes and sclerotic spots (Fig. 6); paler beige markings due to reflective, subcuticular guanine crystals; antero-lateral face of abdomen always with large, humeral patch of reflective guanine crystals (Figs 6A–B, 17A–B).
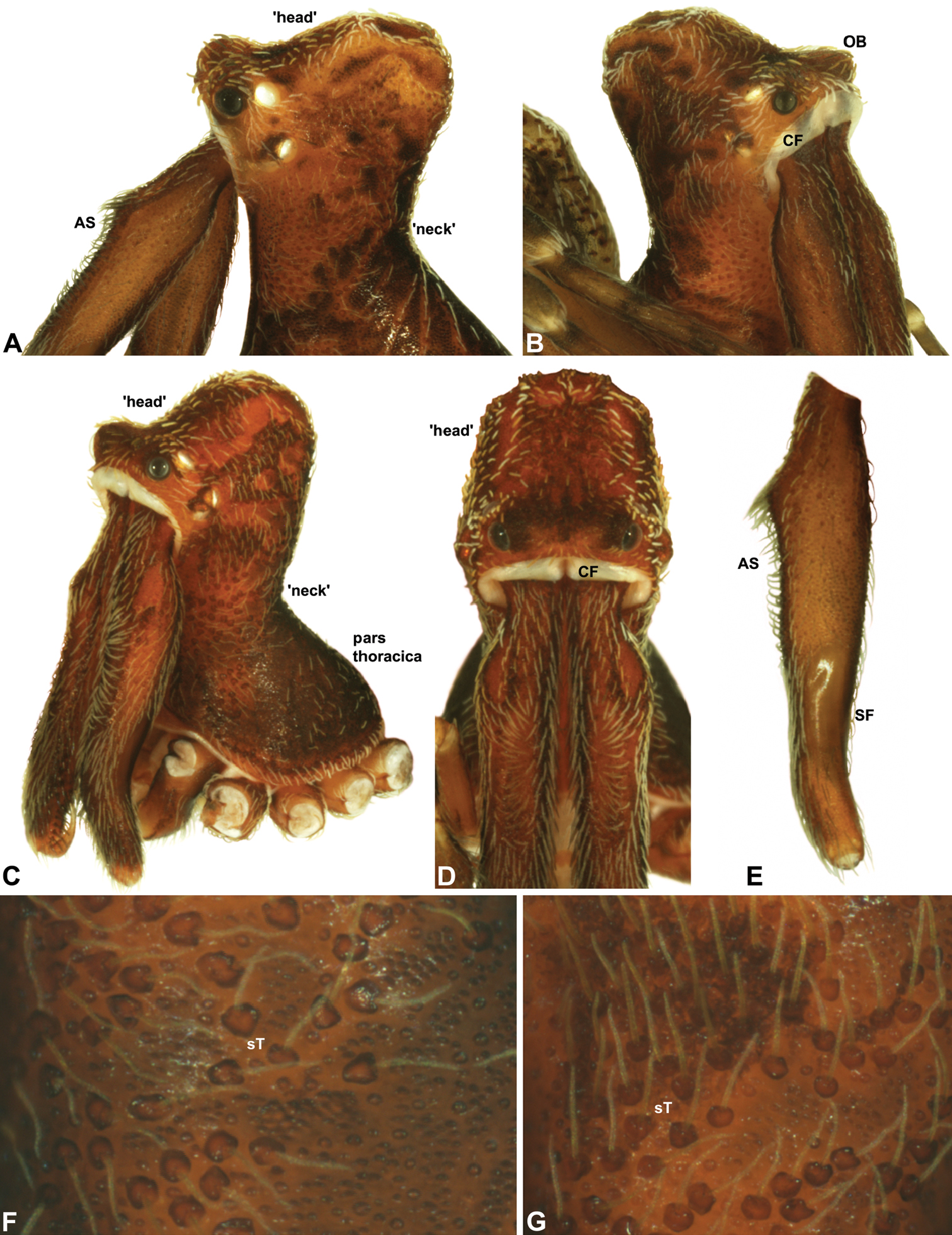
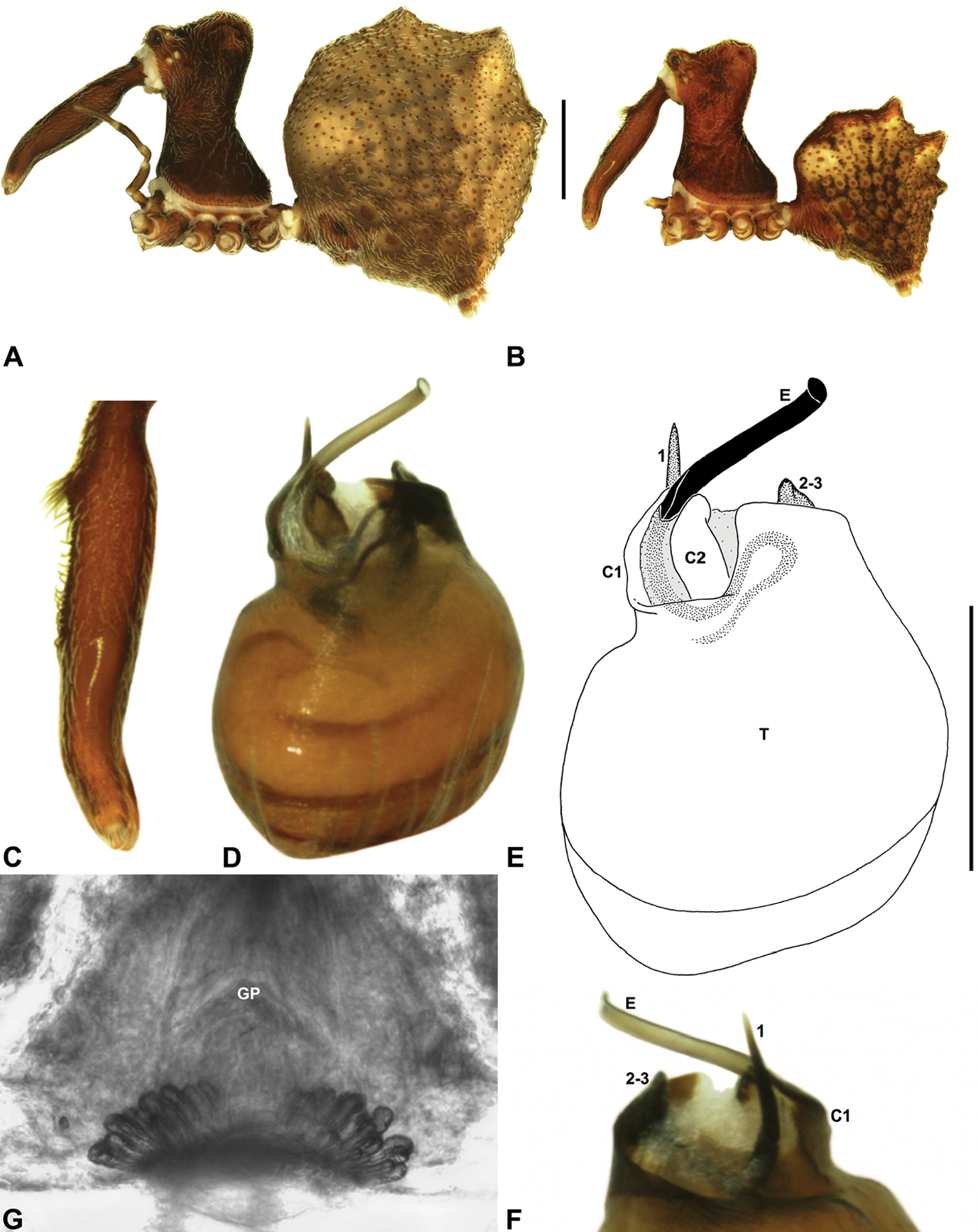
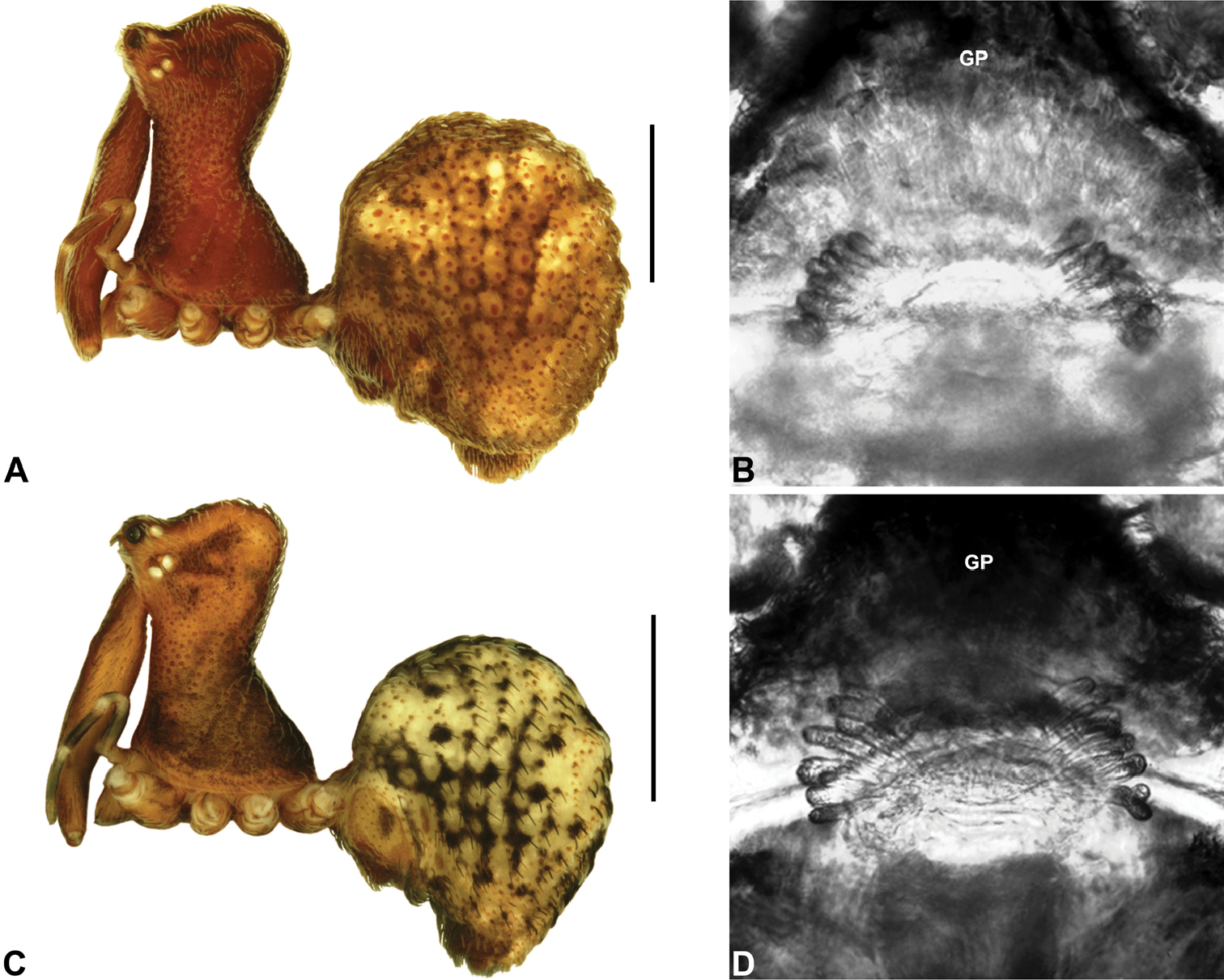
Cephalothorax: Carapace elevated anteriorly (CH/CL ratio usually 1.55–1.75; Fig. 7), with raised, highly modified pars cephalica forming ‘neck’ and bulbous ‘head’ (see Wood 2008; Rix and Harvey 2011) (Figs 5A–D); ‘neck’ with concomitantly long diastema (see Schütt 2002) between cheliceral bases and anterior margin of carapace, fused along entire length with sclerotised cuticle; cheliceral bases emanating from broad, fully-enclosed cheliceral foramen situated at front of ‘head’ (Figs 5B, 5D). Carapace with densely granulate cuticular microstructure, covered in larger setose tubercles arranged in clusters or distinct rows (Figs 5F–G); each tubercle bearing single densely plumose or ciliate seta; setose tubercles largest on ‘neck’ and pars thoracica (Figs 5C, 5F–G). Eight eyes present on anterior margin of ‘head’, in four widely separated diads (Figs 5A–D); AME largest, widely separated, directed antero-laterally on rounded ocular bulge (Fig. 5B); PME situated closely posterior to AME, directed obliquely on postero-lateral side of ocular bulge; lateral eyes contiguous, with shared raised bases, directed ventro-laterally on widest lateral margin of ‘head’ (Figs 5A–D). Sternum longer than wide, covered in setose tubercles; lateral margins separated from dorsal pleural sclerite extending between coxae I–IV. Labium subtriangular, not fused to sternum, directed antero-ventrally at oblique angle to sternum; labrum with pair of divergent projections on anterior surface. Maxillae large, straddling labium and labrum, converging distally; serrula a single row of teeth. Chelicerae very long, spear-like, distally divergent (Figs 5C–E), usually with proximal bulging projection in males (Figs 5A, 5E); both sexes with oval, ectal stridulatory file adjacent to pedipalps (Fig. 5E); males also with tuft (Figs 4B, 5A, 5E, 18B), brush (Figs 14C, 15C) and/or comb (Figs 5C, 16C, 17C) of accessory setae on anterior face of paturon. Chelicerae armed with three rows of peg teeth; anterior (prolateral) row with two peg teeth near tip of fang; posterior (retrolateral) row with single peg tooth near tip of fang; median (prolateral) row with more than 15 peg teeth extending along inner prolateral margin of paturon to near base of fang; median row with approximately eight porrect, comb-like peg teeth adjacent to fang, several larger, flattened, spiniform peg teeth near tip of fang, and additional progressively shorter, spiniform peg teeth along inner paturon; cheliceral retromargin also with four or five true teeth and prominent cheliceral gland mound.
Legs and female pedipalp: Legs (longest to shortest) 1–4–2–3, covered with short plumose setae; spines absent; patella I long, greater than one-third length of femur I. Trichobothria present on tibiae and metatarsi of legs; tibiae I–IV each with two trichobothria; metatarsi I–IV each with single trichobothrium. Tarsi shorter than metatarsi, with three claws; tarsi, metatarsi and distal tibiae of legs I–II usually with ventral and pro-ventral rows of moveable, spatulate setae. Female pedipalp with long, porrect trochanter and small tarsal claw; tibia with two dorsal trichobothria.
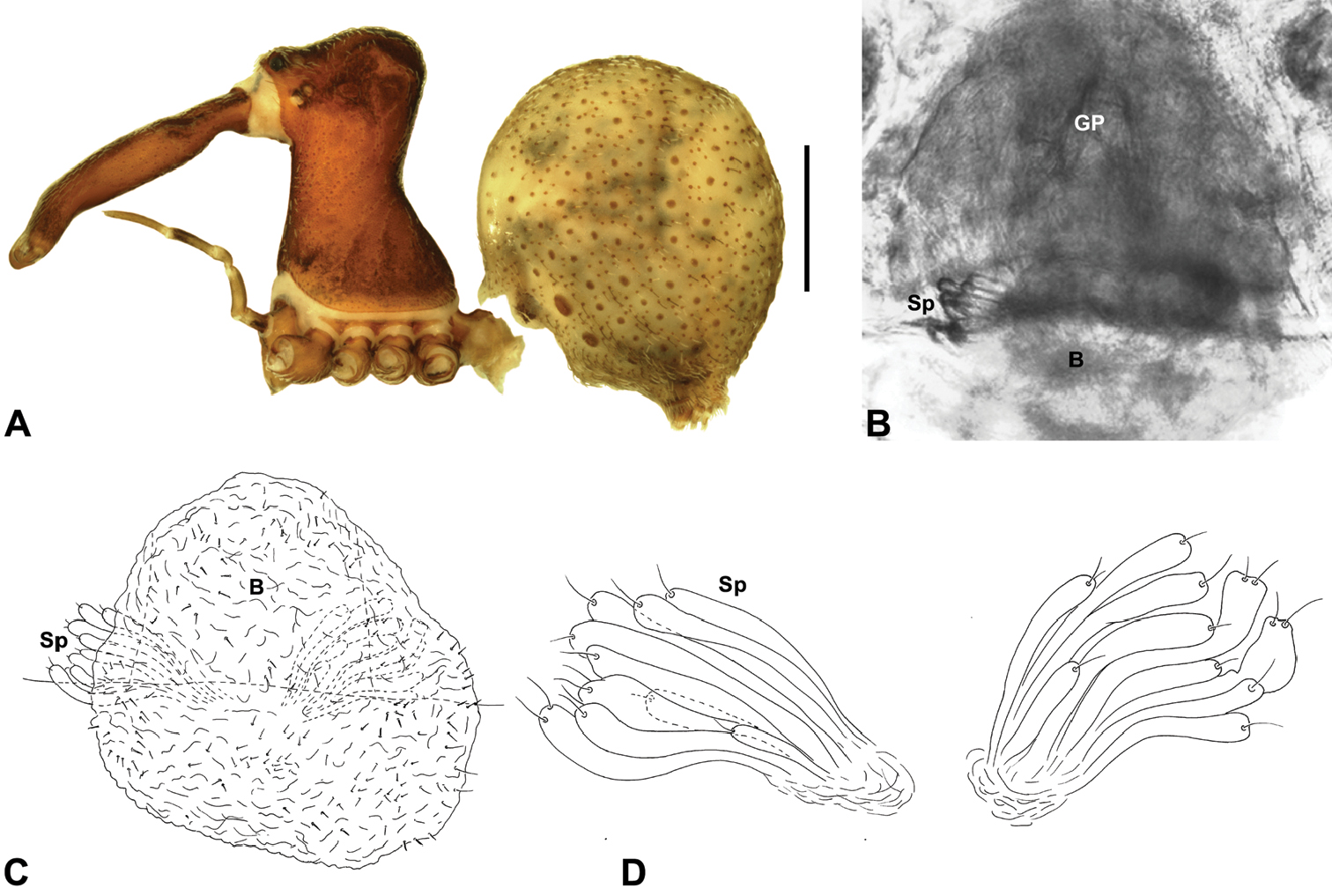
Abdomen: Abdomen arched anteriorly, rounded-subtriangular or spherical in lateral view (Figs 6A–B), sometimes with six large hump-like tubercles on dorsal surface (Figs 6A, 10A–B, 11A–B); cuticle covered with short plumose setae and numerous sclerotic spots (Figs 6C–F). Epigastric region with sclerotised (setose) book lung covers and dorsal and ventral plates surrounding pedicel (Figs 6C–D) (plates fused in males); dorsal pedicel plate with transverse ridges; females with median genital plate and sclerotised lateral sigillae (Fig. 6C); males with broad dorsal scute fused anteriorly to epigastric sclerites, with (Fig. 6A) or usually without (Fig. 6B) additional paired sclerites associated with hump-like tubercles. Six spinnerets, surrounded by thickened cuticle (Fig. 6F); ALS largest, PMS smallest; colulus absent. Posterior pair of divided tracheal spiracles situated closely anterior to spinnerets; males also with epiandrous gland spigots situated closely anterior to epigastric furrow.
Genitalia: Female genitalia haplogyne, with sclerotised, subtriangular genital plate anterior to epigastric furrow (Fig. 6C); internally with gonopore leading to large, membranous bursa (Figs 13B–C; see also Harvey 2002a, fig. 2) overlying two separate, radiating clusters of sclerotised, sausage-shaped anterior spermathecae (Figs 13B–D, 15G, 16G). Male pedipalp with complex, expandable spherical or pyriform bulb (Figs 4D, 10E, 16E, 18D), consisting of smooth tegulum, proximal ‘subtegulum’ and associated tegular groove with basal haematodocha (Fig. 4D); distal tegulum with excavate, rimmed cavity surrounding massive, inflatable haematodochal complex, incorporating distal embolus, basal embolic sclerite, two articulating conductor sclerites and three additional tegular sclerites (Figs 4D, 10E, 18D) (see below). Unexpanded pedipalp with folded, curved embolus abutting paired conductor sclerites (Fig. 10E); other tegular sclerites embedded pro-distally (Fig. 10F); pedipalpal expansion and haematodochal inflation (e.g. see Figs 4D, 18D) resulting in significant conformational changes to length and orientation of embolus, and relative position of tegular sclerites.
As noted by Wood (2008) and Rix and Harvey (2011), the homology of the tegular sclerites among archaeid genera remains unclear. Rix and Harvey (2011) used a numbering system for comparing the moveable tegular sclerites among species of Austrarchaea from mid-eastern Australia, identifying four separate sclerites (TS 1, 2, 2a and 3) according to their relative position within the unexpanded tegular cavity (see Fig. 4C). These four sclerites can be broadly homologised with the tegular sclerites of species of Zephyrarchaea, although at least one sclerite appears to be absent or otherwise highly modified in the latter, with no evidence for an interlocking, differentiated TS 2–2a complex (as in Austrarchaea; Fig. 4C cf. Fig. 4D). Tegular sclerite 1 (TS 1) is a prominent and strongly developed process in all species of Zephyrarchaea (Figs 4D, 10F, 16F), originating proventrally adjacent to the base of conductor sclerite 1. Two additional tegular sclerites (here labelled collectively TS 2–3) are closely contiguous and not easily distinguished in the unexpanded state, usually embedded pro-distally adjacent to the retro-distal rim of the tegulum. The larger of these two sclerites, presumably homologous to tegular sclerite 3 (TS 3) in species of Austrarchaea, has a shorter and broader, more plate-like morphology relative to TS 1, and is usually (but not always) visible as a pointed projection beyond the retro-distal rim of the tegulum (Figs 10E, 16E).
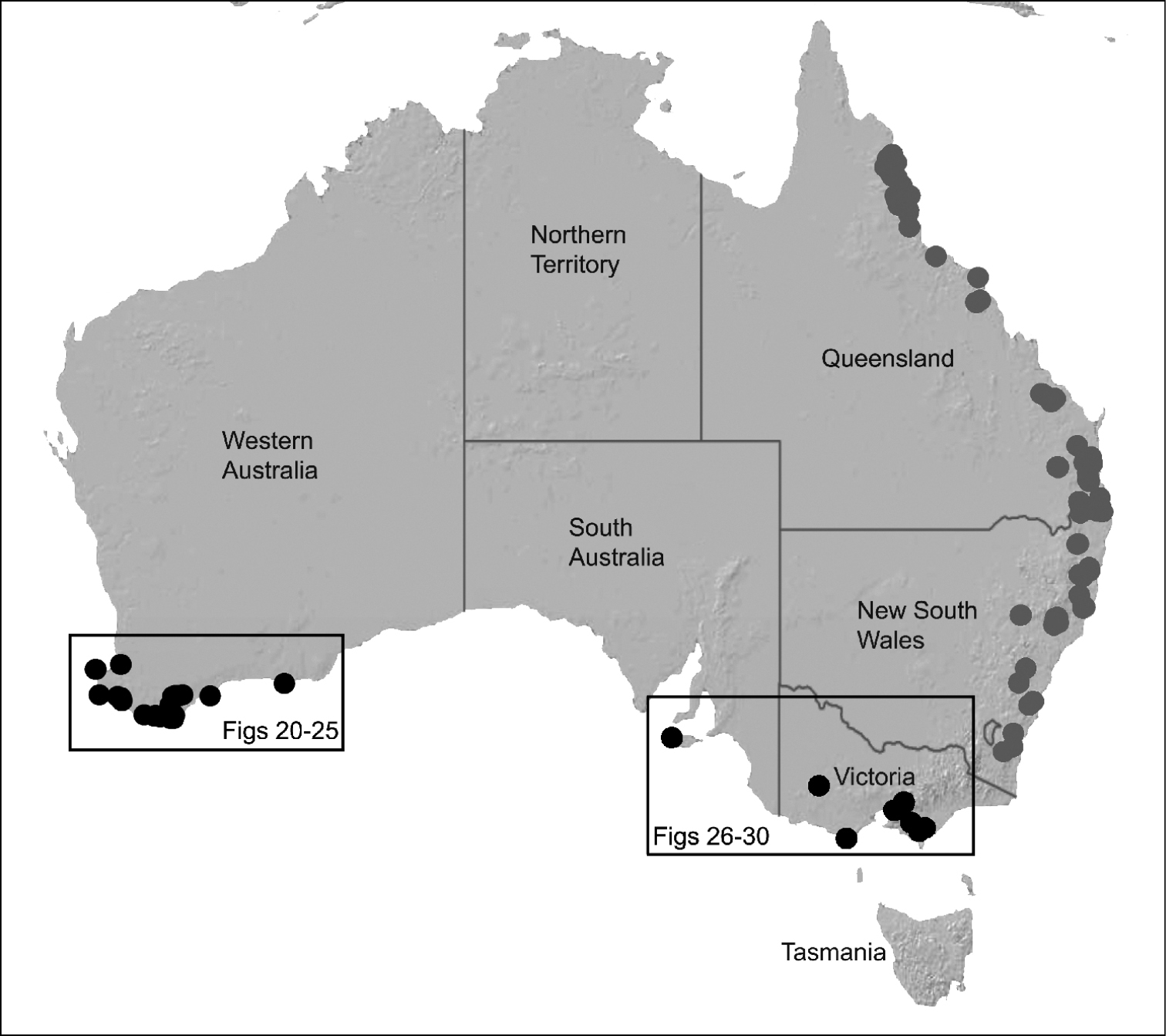
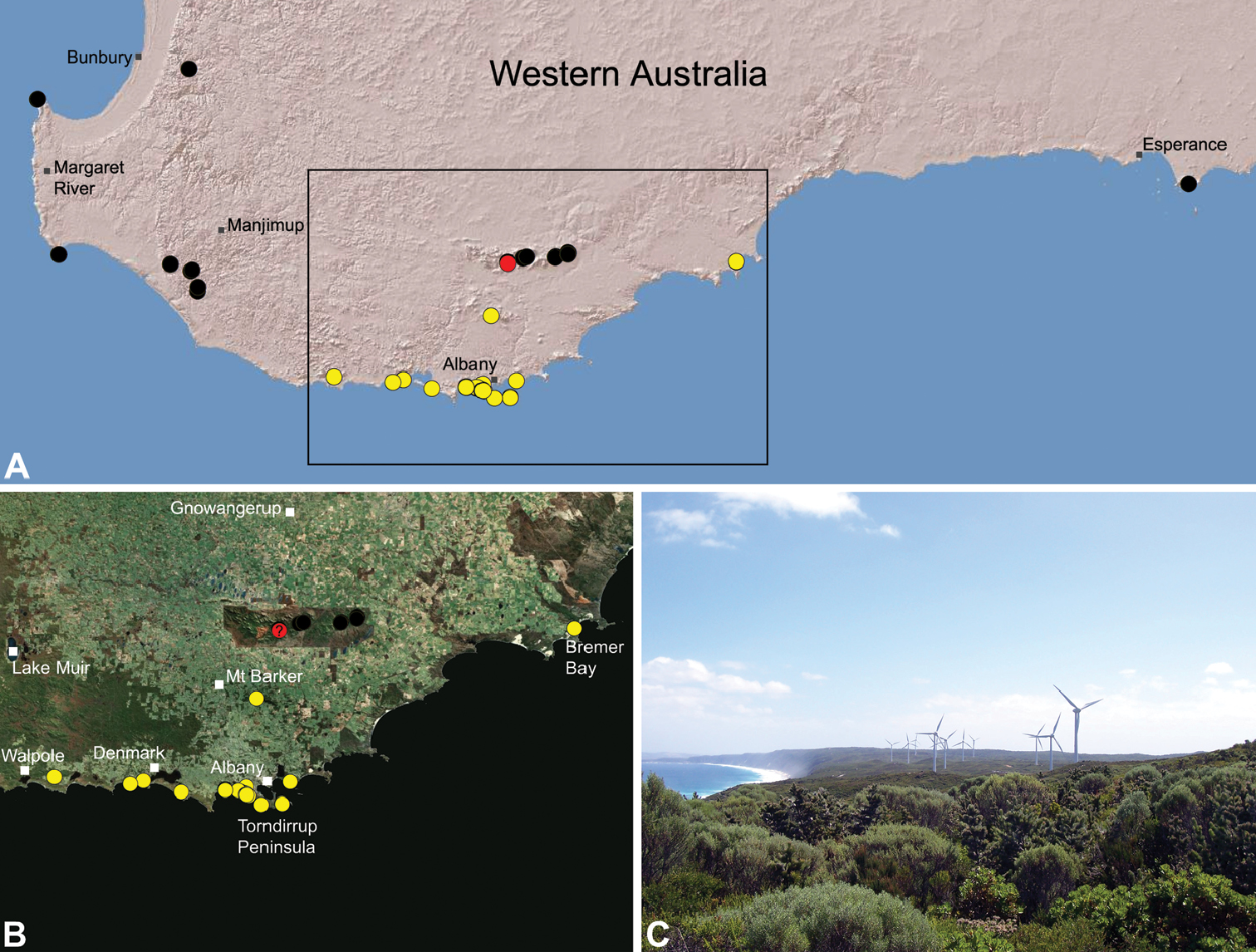
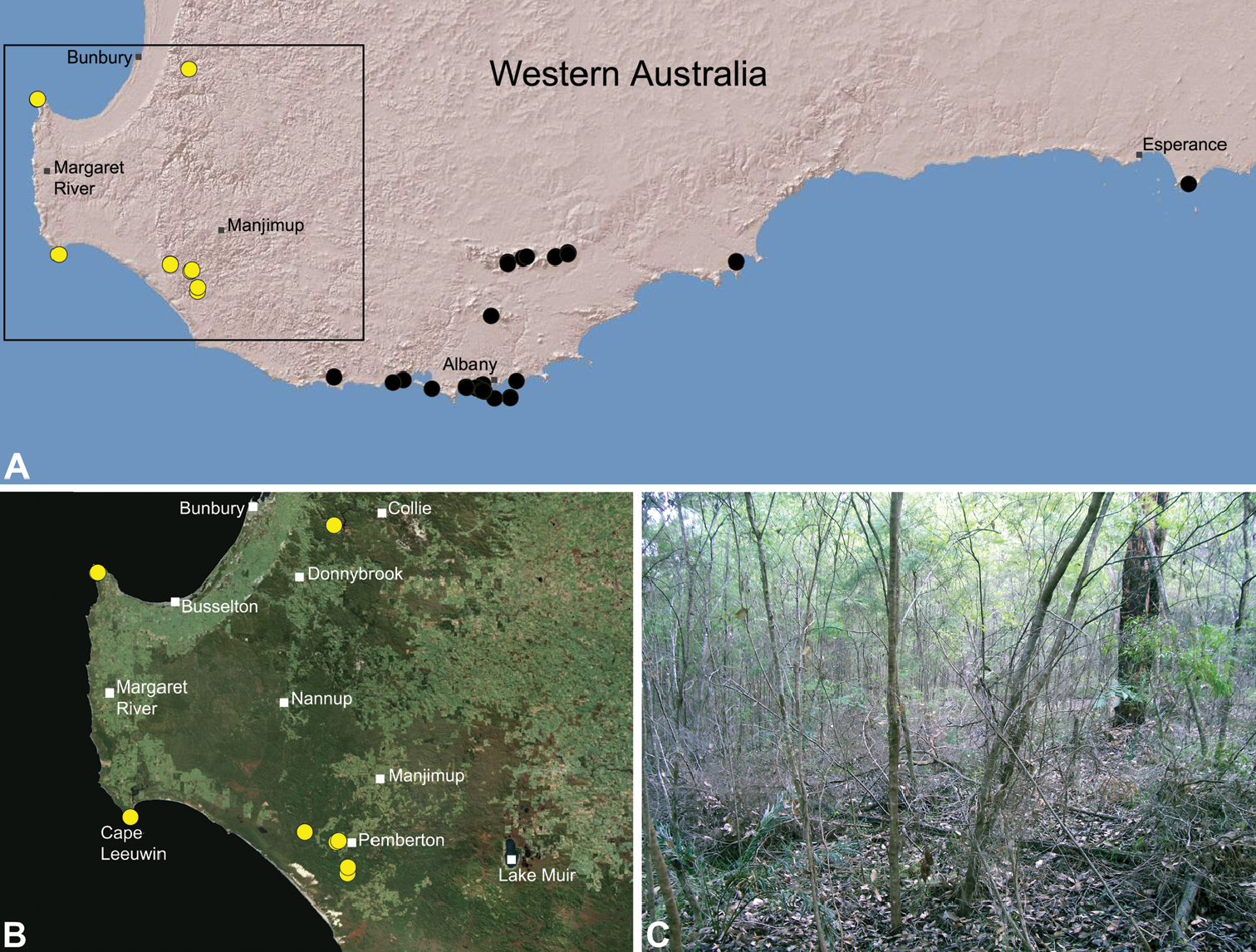
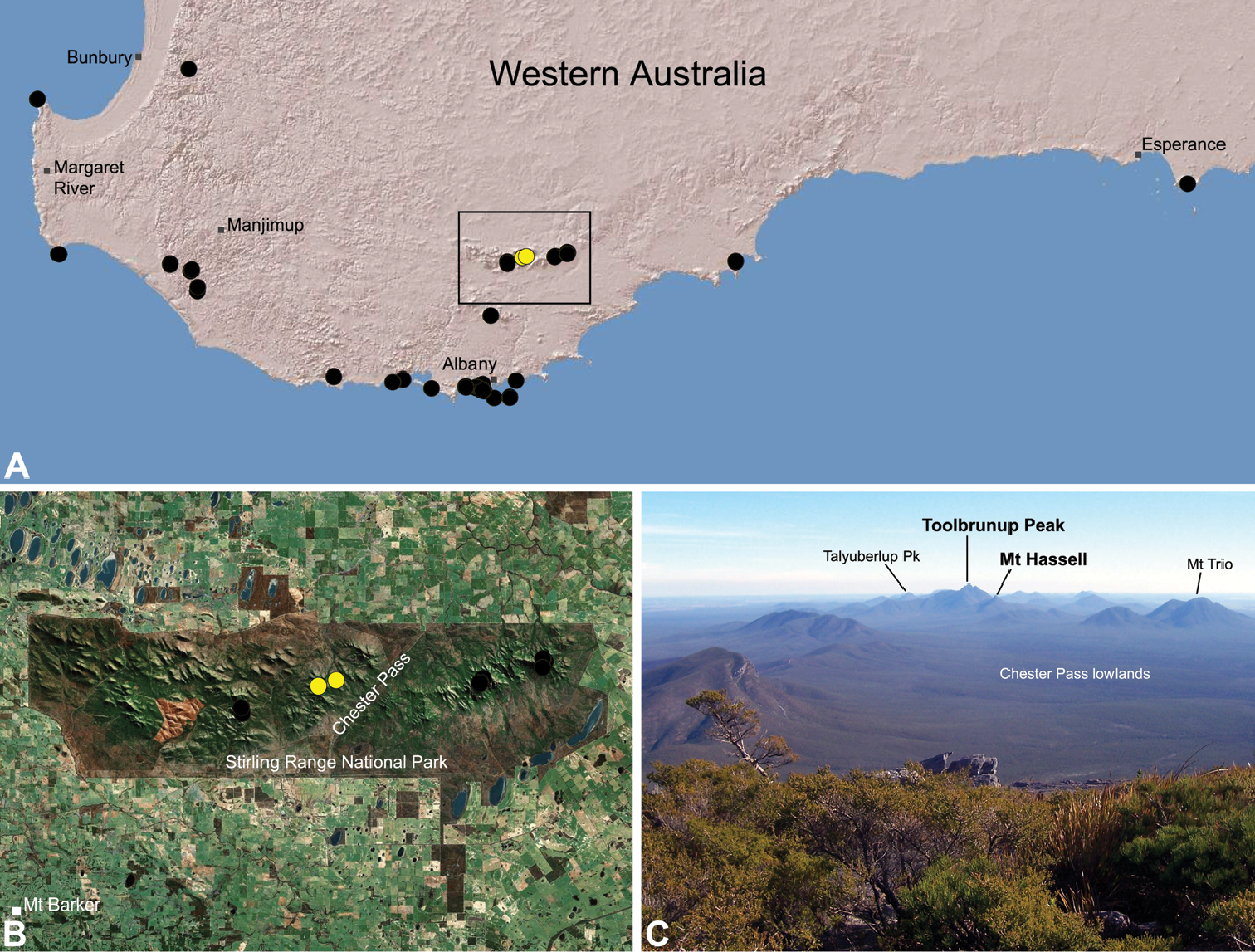
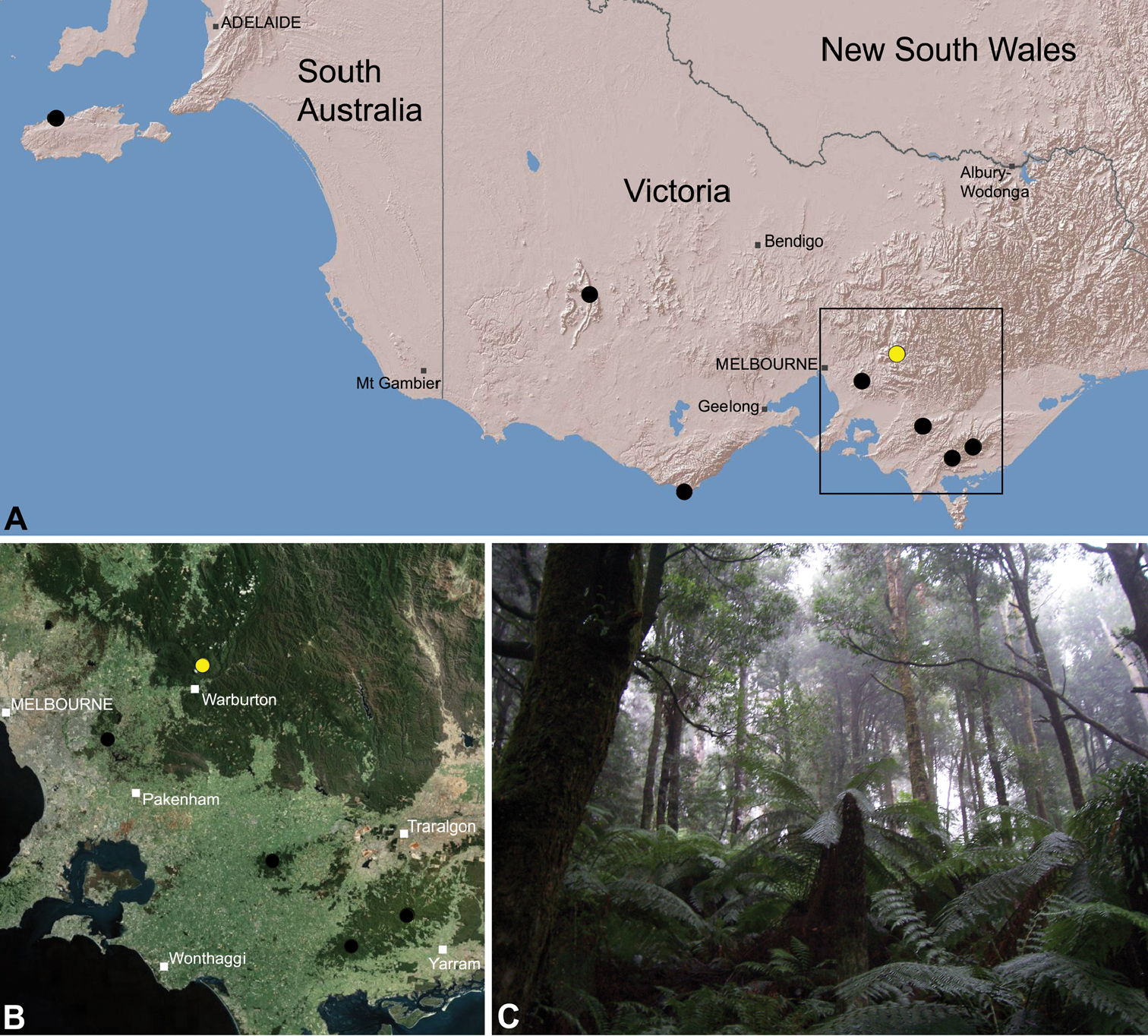
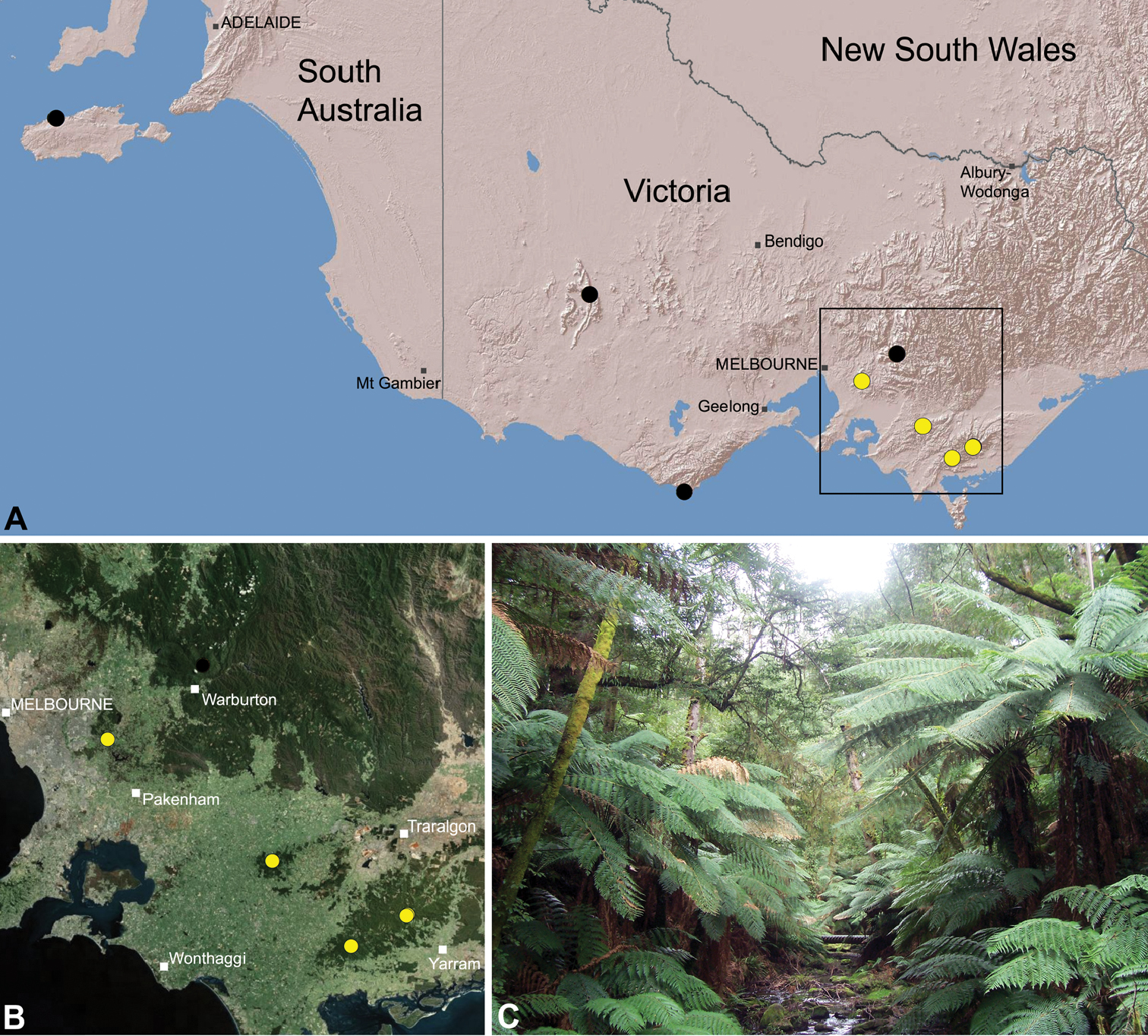
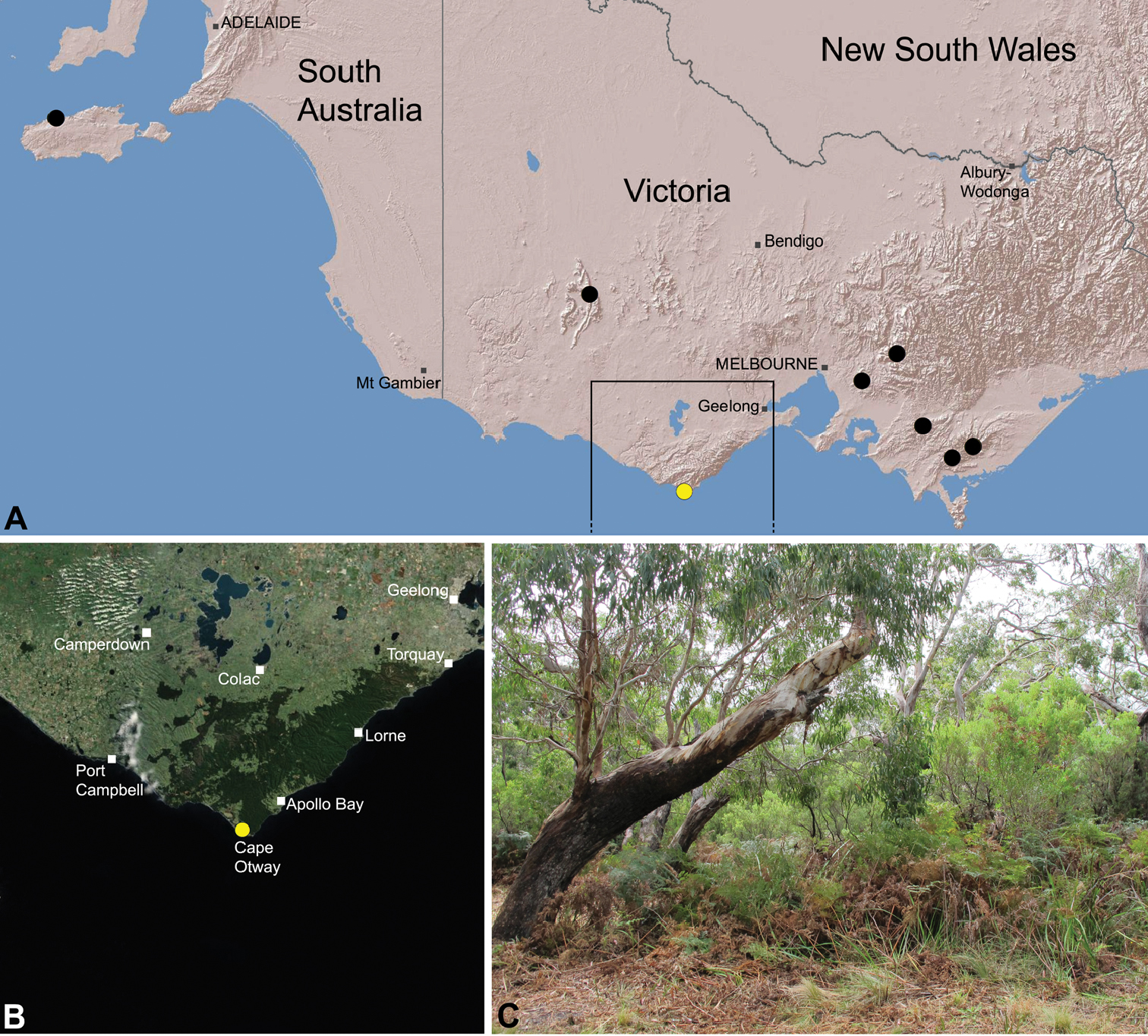
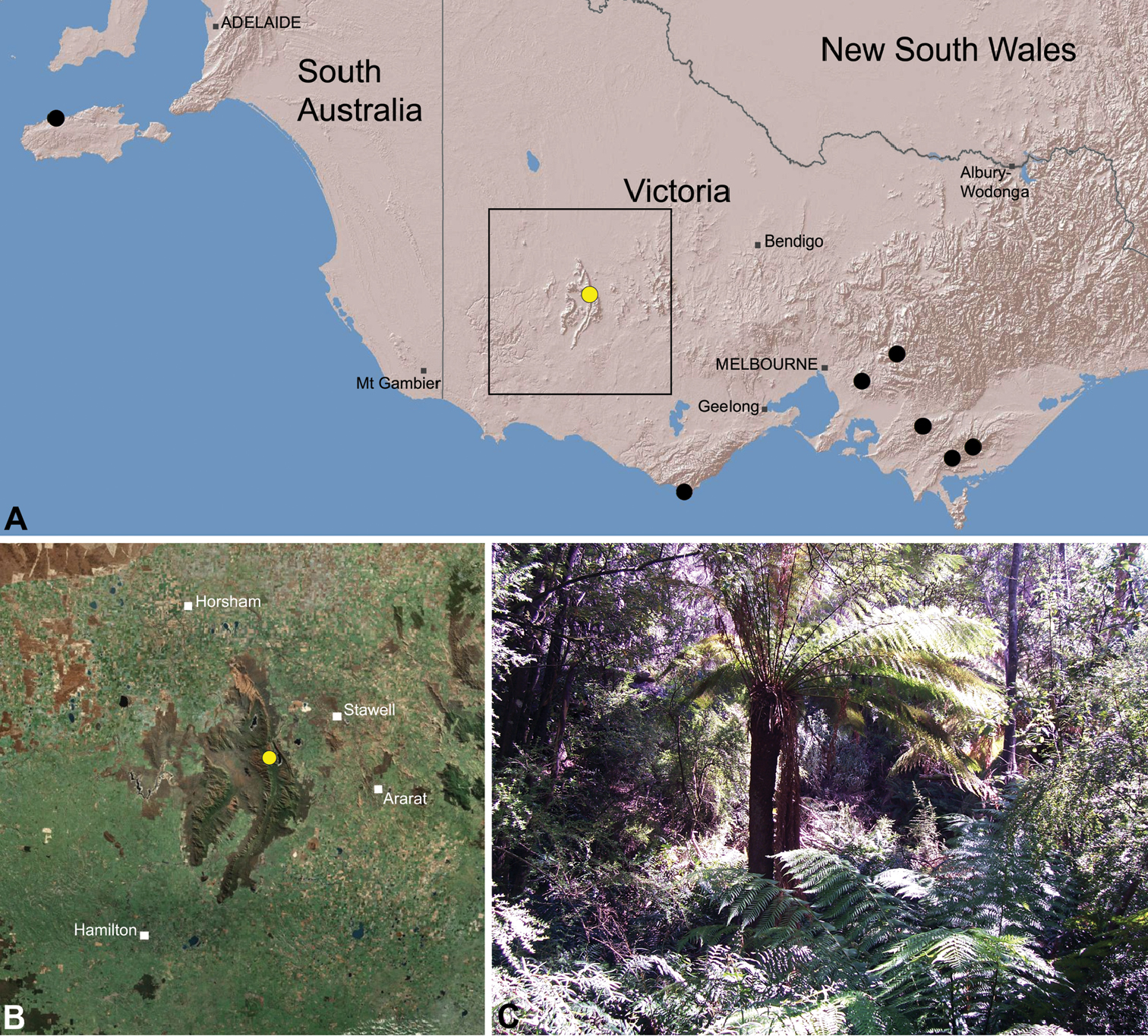
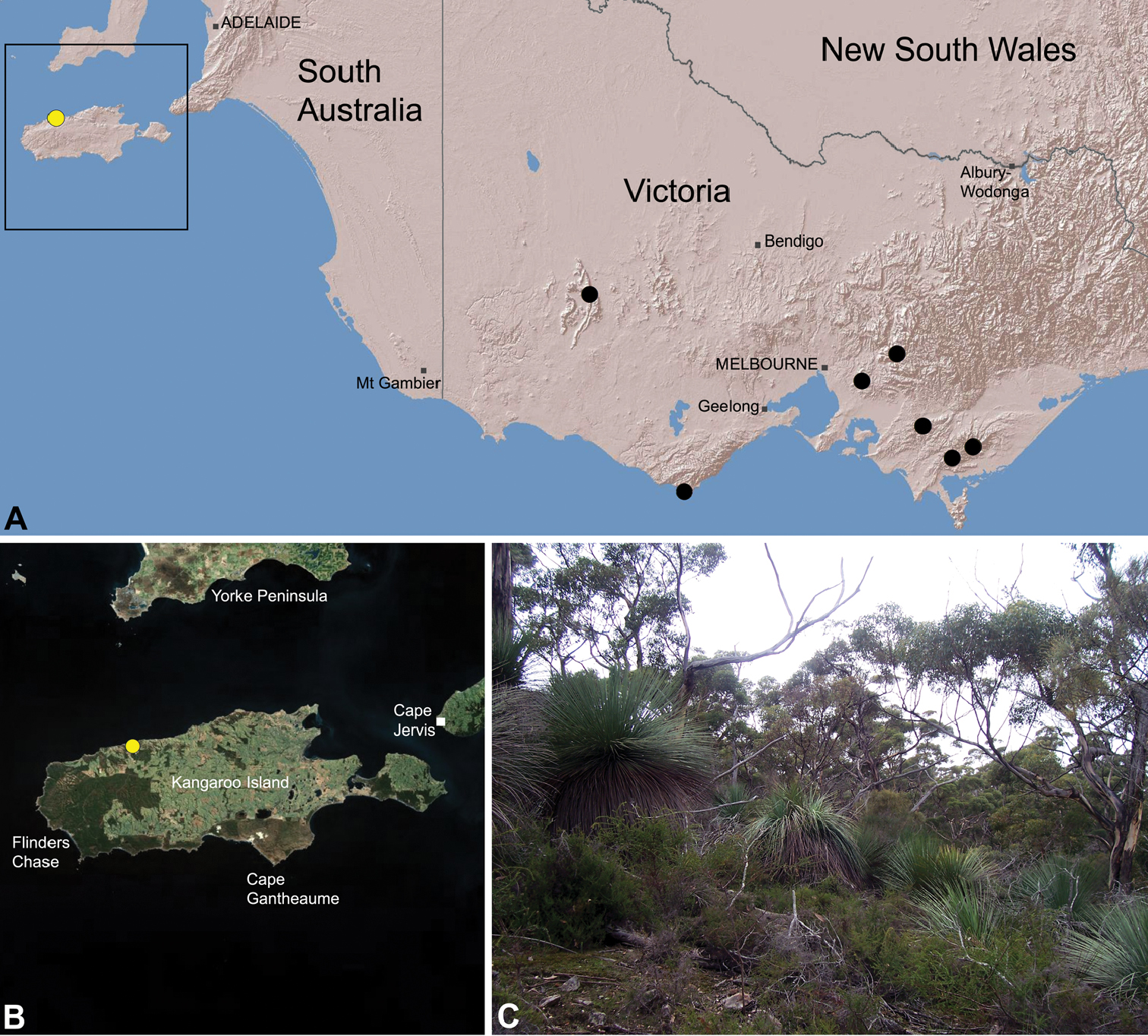
Species of Zephyrarchaea occur in mesic habitats throughout southern Western Australia, South Australia and Victoria (Fig. 2), usually in coastal (Figs 20C, 22C), sub-coastal or montane (Figs 23C, 24C) temperate heathlands, but also in wet eucalypt forests (Figs 21C, 29C) and temperate rainforests (Figs 26C, 27C). In Victoria they occur along the Great Dividing Range, from Grampians National Park and the Otway Range in south-western Victoria east to the Yarra and Strzelecki Ranges east of Melbourne (Figs 26–29). In South Australia they occur on Kangaroo Island, at a single known locality north of Flinders Chase (Fig. 30). In south-western Western Australia they occur in the southern high rainfall and south-eastern coastal provinces (see Hopper and Gioia 2004; Figs 20–25), from the Wellington and Leeuwin-Naturaliste National Parks (near Bunbury) east to Cape Le Grand National Park, with outlying populations in the Porongurup and Stirling Range National Parks.
Two described species – Zephyrarchaea mainae (Platnick, 1991b) and Zephyrarchaea robinsi (Harvey, 2002a) – and the nine new species from southern Australia: Zephyrarchaea austini sp. n., Zephyrarchaea barrettae sp. n., Zephyrarchaea grayi sp. n., Zephyrarchaea janineae sp. n., Zephyrarchaea marae sp. n., Zephyrarchaea marki sp. n., Zephyrarchaea melindae sp. n., Zephyrarchaea porchi sp. n. and Zephyrarchaea vichickmani sp. n. The previously described species Archaea hickmani Butler, 1929 is here recognised as a nomen dubium.
The genus Zephyrarchaea forms a monophyletic and highly divergent clade sister to all other Archaeidae from mid-eastern and north-eastern Australia (see Rix and Harvey 2011, 2012; Fig. 3). Three main lineages have been recognised within the genus, for species from south-eastern Australia (South Australia and Victoria), from the Stirling Range National Park and from elsewhere in south-western Western Australia (see Rix and Harvey 2012; Fig. 3). The genus is not known to occur north or east of the Australian Alps, which may be a vicariant biogeographic barrier between populations of Zephyrarchaea and Austrarchaea.
Note that males of Zephyrarchaea austini sp. n., Zephyrarchaea grayi sp. n. and Zephyrarchaea robinsi are unknown; females of Zephyrarchaea marki sp. n. and Zephyrarchaea porchi sp. n. are unknown.
| 1 | Males | 2 |
| – | Females | 9 |
| 2 | Abdomen with six pronounced dorsal hump-like tubercles (HT 1–6), in three pairs, HT 3–6 each with small dorsal sclerite posterior to dorsal scute (Figs 6A, 10B, 11B) | 3 |
| – | Abdomen spherical, or nearly so, without pronounced hump-like tubercles and without additional sclerites posterior to dorsal scute (Figs 1B, 1D, 6B, 12A, 14B, 15B, 16B, 17B, 18A) | 4 |
| 3 | Tegular sclerite 1 (TS 1) with flattened, rounded apex (Figs 10D–F) | Zephyrarchaea mainae (Platnick, 1991b) |
| – | Tegular sclerite 1 (TS 1) with more tapered, spiniform apex (Figs 11D–F) | Zephyrarchaea janineae sp. n. |
| 4 | Chelicerae with proximal bulging projection bearing tuft or brush of accessory setae (Figs 12B, 14C, 15C, 18B) | 5 |
| – | Chelicerae without tuft of accessory setae on proximal bulging projection (Figs 16C, 17C) | 8 |
| 5 | Tegular sclerites 2–3 (TS 2–3) not projecting beyond retro-distal margin of tegulum (Figs 14D–E, 15D–E) | 6 |
| – | Tegular sclerites 2–3 (TS 2–3) projecting well beyond retro-distal margin of tegulum (Figs 12C–D, 18C–D) | 7 |
| 6 | Anterior margin of diastema adjacent to ‘neck’ almost straight, only slightly concave in lateral view (Fig. 14B) | Zephyrarchaea melindae sp. n. |
| – | Anterior margin of diastema adjacent to ‘neck’ curved, strongly concave in lateral view (Fig. 15B) | Zephyrarchaea barrettae sp. n. |
| 7 | Proximal bulging projection on chelicerae strongly protuberant (Fig. 12B); dorsal scute on abdomen extending posteriorly (in lateral view) to roughly in-line with epigastric furrow (Fig. 12A) | Zephyrarchaea marki sp. n. |
| – | Proximal bulging projection on chelicerae indistinct, only slightly protuberant (Fig. 18B); dorsal scute on abdomen larger, extending posteriorly (in lateral view) to beyond line of epigastric furrow (Fig. 18A) | Zephyrarchaea porchi sp. n. |
| 8 | Conductor sclerite 2 (C2) relatively slender, with sinuous, S-shaped proximal portion (Figs 17D–E); anterior margin of diastema adjacent to ‘neck’ slightly convex in lateral view (Fig. 17B) | Zephyrarchaea marae sp. n. |
| – | Conductor sclerite 2 (C2) without sinuous, S-shaped proximal portion (Figs 16D–E); anterior margin of diastema adjacent to ‘neck’ slightly concave in lateral view (Fig. 16B) | Zephyrarchaea vichickmani sp. n. |
| 9 | Abdomen with six pronounced dorsal hump-like tubercles (HT 1–6), in three pairs (Figs 1C, 1E–F, 10A, 11A) | 10 |
| – | Abdomen spherical, or nearly so, without pronounced dorsal hump-like tubercles (Figs 1A, 13A, 14A, 15A, 16A, 17A, 19A, 19C) | 11 |
| 10 | ‘Head’ not strongly elevated dorsally, post-ocular ratio < 0.25 (Fig. 9E); highest point of pars cephalica (HPC) near posterior third of ‘head’, ratio of HPC to post-ocular length ≤ 0.66 (Fig. 9E) | Zephyrarchaea mainae (Platnick, 1991b)* |
| – | ‘Head’ more strongly elevated dorsally, post-ocular ratio ≥ 0.25 (Fig. 9F); highest point of pars cephalica (HPC) approaching posterior quarter of ‘head’, ratio of HPC to post-ocular length > 0.66 (Fig. 9F) | Zephyrarchaea janineae sp. n.* |
| 11 | Carapace with strongly concave post-ocular depression in lateral view (Figs 9A–D) | 12 |
| – | Carapace with shallow post-ocular depression in lateral view (Figs 9G–I) | 14 |
| 12 | Body size small, carapace length < 1.10 (Fig. 7); carapace relatively short, CH/CL ratio < 1.70 (Figs 7, 19C) | Zephyrarchaea austini sp. n. |
| – | Body size larger, carapace length > 1.10 (Fig. 7); carapace taller, CH/CL ratio ≥ 1.70 (Figs 7, 19A) | 13 |
| 13 | ‘Head’ not strongly elevated dorsally, post-ocular ratio < 0.25 (Fig. 9C); highest point of pars cephalica (HPC) approaching middle of ‘head’, ratio of HPC to post-ocular length < 0.60 (Fig. 9C) | Zephyrarchaea grayi sp. n. |
| – | ‘Head’ more strongly elevated dorsally, post-ocular ratio ≥ 0.25 (Figs 9A–B); highest point of pars cephalica (HPC) approaching posterior third of ‘head’, ratio of HPC to post-ocular length > 0.60 (Figs 9A–B) | Zephyrarchaea marae sp. n./Zephyrarchaea vichickmani sp. n.** |
| 14 | Carapace relatively short, CH/CL ratio < 1.70 (Figs 7, 13A) | Zephyrarchaea robinsi (Harvey, 2002a) |
| – | Carapace taller, CH/CL ratio > 1.70 (Figs 7, 14A, 15A) | 15 |
| 15 | Anterior margin of diastema adjacent to ‘neck’ straight, almost perpendicular in lateral view (Fig. 14A) | Zephyrarchaea melindae sp. n. |
| – | Anterior margin of diastema adjacent to ‘neck’ slightly curved, concave in lateral view (Fig. 15A) | Zephyrarchaea barrettae sp. n. |
| 1 | Males | 2 |
| – | Females | 9 |
| 2 | Abdomen with six pronounced dorsal hump-like tubercles (HT 1–6), in three pairs, HT 3–6 each with small dorsal sclerite posterior to dorsal scute (Figs 6A, 10B, 11B) | 3 |
| – | Abdomen spherical, or nearly so, without pronounced hump-like tubercles and without additional sclerites posterior to dorsal scute (Figs 1B, 1D, 6B, 12A, 14B, 15B, 16B, 17B, 18A) | 4 |
| 3 | Tegular sclerite 1 (TS 1) with flattened, rounded apex (Figs 10D–F) | Zephyrarchaea mainae (Platnick, 1991b) |
| – | Tegular sclerite 1 (TS 1) with more tapered, spiniform apex (Figs 11D–F) | Zephyrarchaea janineae sp. n. |
| 4 | Chelicerae with proximal bulging projection bearing tuft or brush of accessory setae (Figs 12B, 14C, 15C, 18B) | 5 |
| – | Chelicerae without tuft of accessory setae on proximal bulging projection (Figs 16C, 17C) | 8 |
| 5 | Tegular sclerites 2–3 (TS 2–3) not projecting beyond retro-distal margin of tegulum (Figs 14D–E, 15D–E) | 6 |
| – | Tegular sclerites 2–3 (TS 2–3) projecting well beyond retro-distal margin of tegulum (Figs 12C–D, 18C–D) | 7 |
| 6 | Anterior margin of diastema adjacent to ‘neck’ almost straight, only slightly concave in lateral view (Fig. 14B) | Zephyrarchaea melindae sp. n. |
| – | Anterior margin of diastema adjacent to ‘neck’ curved, strongly concave in lateral view (Fig. 15B) | Zephyrarchaea barrettae sp. n. |
| 7 | Proximal bulging projection on chelicerae strongly protuberant (Fig. 12B); dorsal scute on abdomen extending posteriorly (in lateral view) to roughly in-line with epigastric furrow (Fig. 12A) | Zephyrarchaea marki sp. n. |
| – | Proximal bulging projection on chelicerae indistinct, only slightly protuberant (Fig. 18B); dorsal scute on abdomen larger, extending posteriorly (in lateral view) to beyond line of epigastric furrow (Fig. 18A) | Zephyrarchaea porchi sp. n. |
| 8 | Conductor sclerite 2 (C2) relatively slender, with sinuous, S-shaped proximal portion (Figs 17D–E); anterior margin of diastema adjacent to ‘neck’ slightly convex in lateral view (Fig. 17B) | Zephyrarchaea marae sp. n. |
| – | Conductor sclerite 2 (C2) without sinuous, S-shaped proximal portion (Figs 16D–E); anterior margin of diastema adjacent to ‘neck’ slightly concave in lateral view (Fig. 16B) | Zephyrarchaea vichickmani sp. n. |
| 9 | Abdomen with six pronounced dorsal hump-like tubercles (HT 1–6), in three pairs (Figs 1C, 1E–F, 10A, 11A) | 10 |
| – | Abdomen spherical, or nearly so, without pronounced dorsal hump-like tubercles (Figs 1A, 13A, 14A, 15A, 16A, 17A, 19A, 19C) | 11 |
| 10 | ‘Head’ not strongly elevated dorsally, post-ocular ratio < 0.25 (Fig. 9E); highest point of pars cephalica (HPC) near posterior third of ‘head’, ratio of HPC to post-ocular length ≤ 0.66 (Fig. 9E) | Zephyrarchaea mainae (Platnick, 1991b)* |
| – | ‘Head’ more strongly elevated dorsally, post-ocular ratio ≥ 0.25 (Fig. 9F); highest point of pars cephalica (HPC) approaching posterior quarter of ‘head’, ratio of HPC to post-ocular length > 0.66 (Fig. 9F) | Zephyrarchaea janineae sp. n.* |
| 11 | Carapace with strongly concave post-ocular depression in lateral view (Figs 9A–D) | 12 |
| – | Carapace with shallow post-ocular depression in lateral view (Figs 9G–I) | 14 |
| 12 | Body size small, carapace length < 1.10 (Fig. 7); carapace relatively short, CH/CL ratio < 1.70 (Figs 7, 19C) | Zephyrarchaea austini sp. n. |
| – | Body size larger, carapace length > 1.10 (Fig. 7); carapace taller, CH/CL ratio ≥ 1.70 (Figs 7, 19A) | 13 |
| 13 | ‘Head’ not strongly elevated dorsally, post-ocular ratio < 0.25 (Fig. 9C); highest point of pars cephalica (HPC) approaching middle of ‘head’, ratio of HPC to post-ocular length < 0.60 (Fig. 9C) | Zephyrarchaea grayi sp. n. |
| – | ‘Head’ more strongly elevated dorsally, post-ocular ratio ≥ 0.25 (Figs 9A–B); highest point of pars cephalica (HPC) approaching posterior third of ‘head’, ratio of HPC to post-ocular length > 0.60 (Figs 9A–B) | Zephyrarchaea marae sp. n./Zephyrarchaea vichickmani sp. n.** |
| 14 | Carapace relatively short, CH/CL ratio < 1.70 (Figs 7, 13A) | Zephyrarchaea robinsi (Harvey, 2002a) |
| – | Carapace taller, CH/CL ratio > 1.70 (Figs 7, 14A, 15A) | 15 |
| 15 | Anterior margin of diastema adjacent to ‘neck’ straight, almost perpendicular in lateral view (Fig. 14A) | Zephyrarchaea melindae sp. n. |
| – | Anterior margin of diastema adjacent to ‘neck’ slightly curved, concave in lateral view (Fig. 15A) | Zephyrarchaea barrettae sp. n. |
* Females of Zephyrarchaea mainae and Zephyrarchaea janineae sp. n. are very similar morphologically, with only subtle morphometric differences in the shape of the carapace; male specimens or nucleotide sequences are recommended to accurately identify these closely related species.
** Females of Zephyrarchaea vichickmani sp. n. and Zephyrarchaea marae sp. n. are essentially indistinguishable morphologically, with male specimens or nucleotide sequences required to identify these closely related sister-species.
http://species-id.net/wiki/Zephyrarchaea_mainae
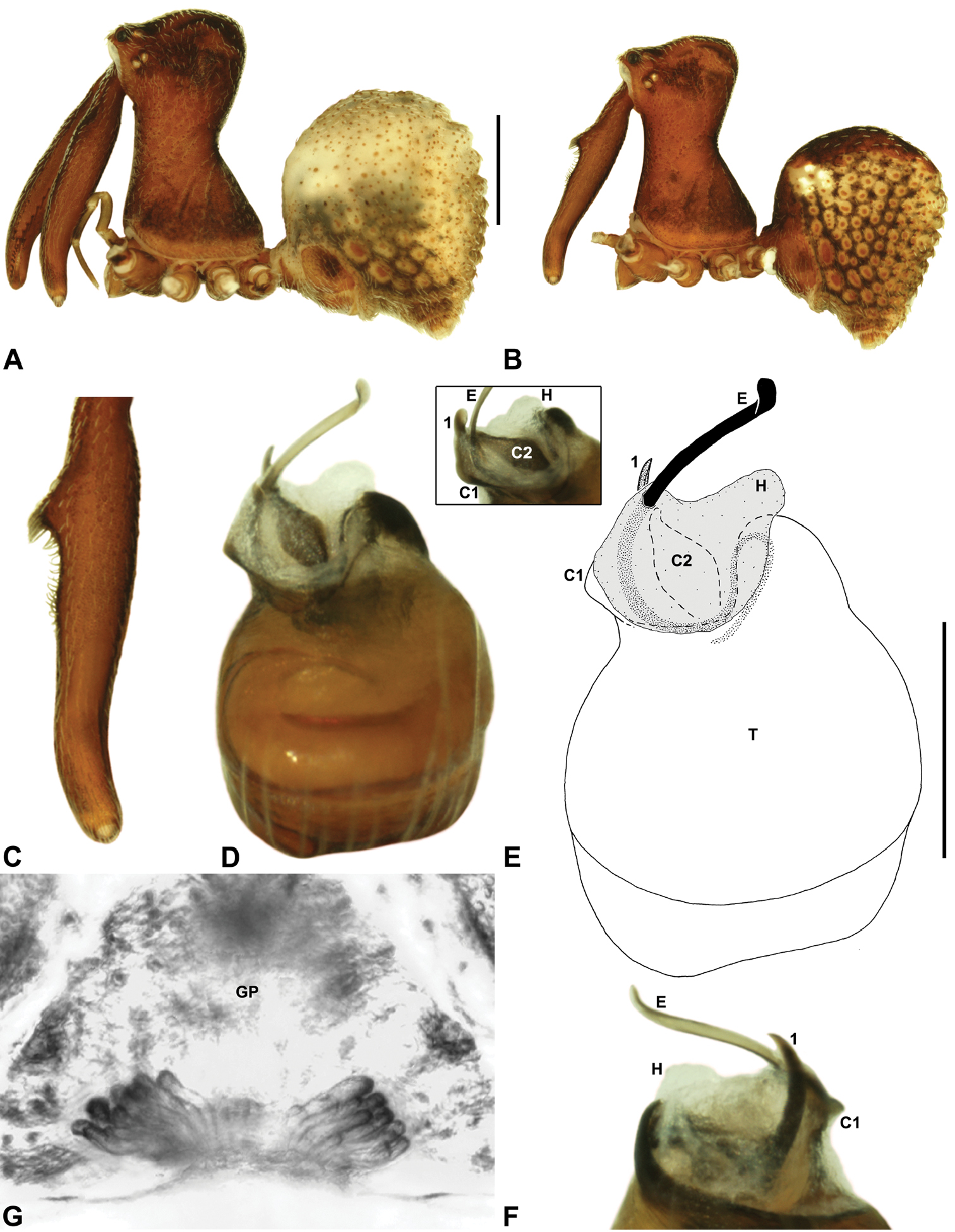
Albany Assassin SpiderFigs 1E–F, 5A–B, 5E, 6A, 6C–D, 6F, 8G, 9E, 10, 20
Holotype male. Torndirrup National Park (probably near end of Eclipse Island Road), Western Australia, Australia, pitfall trap, 1–6.VI.1983, P. Dyer, J. Lyon (WAM T17683).
Paratypes. Allotype female, same data as holotype except 3–8.X.1983 (WAM T17684).
AUSTRALIA: Western Australia: Torndirrup National Park: same data as holotype except 2–9.XI.1983, 1 juvenile (WAM T17682); next to carpark at end of Salmon Hole Road, 35°06'07"S, 117°58'03"E, sifting elevated leaf litter under Agonis, 14.III.2008, M. Rix, M. Harvey, 1♀, 2 juveniles (WAM T89566DNA: TO-1-J/TO-2-J); same data except 30 April 2008, 1♀ (AMNH); same data, 1♀ (QMB S91206); same data, 1 juvenile (WAM T89567DNA: TO-171-J); next to carpark at base of Isthmus Hill, 35°05'55"S, 117°58'02"E, sifting elevated leaf litter in coastal Agonis and eucalypt grove, 14.III.2008, M. Rix, M. Harvey, 2 juveniles (WAM T89568DNA: TO-3-J/TO-4-J). Bremer Bay: Wellstead Road, S. of Bremer Bay, near Yate Place, 34°24'10"S, 119°22'42"E, sifting elevated leaf litter in Agonis grove, 19.VI.2010, M. Rix, J.D. Roberts, 1♂, 1♀ (WAM T118984); same data except 21.IV.2009, M. Rix, 1♂, 1 juvenile (WAM T97463DNA: BB-147-J); same data except 2.V.2008, M. Rix, M. Harvey, J. Newell, 1♀, 1 juvenile (WAM T89563DNA: BB-34-F/BB-35-J). Denmark Region: Gilge Road, 15 km SE. of Denmark, 35°03'15"S, 117°28'49"E, sifting elevated leaf litter in remnant Karri grove with Agonis, 16.III.2008, M. Rix, M. Harvey, 1♀, 1 juvenile (WAM T89577DNA: GI-14-F/GI-15-J); Mount Hallowell, 7 km SW. of Denmark, 35°00'34"S, 117°18'04"E, beating grass clumps in Karri forest, 6.XI.2007, M. Moir, D. Jolly, 1 juvenile (WAM T78901); Mount Hallowell, 7 km SW. of Denmark, 35°00'38"S, 117°17'57"E, beating grass clump in Karri forest, 19.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89590DNA: MH-30-J). Gull Rock National Park: end of Ledge Point Road, near carpark, 35°00'51"S, 118°00'23"E, sifting elevated leaf litter under Agonis, 17.III.2008, M. Rix, M. Harvey, 1♀, 1 juvenile (WAM T89578DNA: GR-16-F/GR-17-J). Mutton Bird Point: end of Mutton Bird Road, 35°02'53"S, 117°41'42"E, sifting elevated leaf litter under Agonis, 19.VI.2010, M. Rix, J.D. & B. Roberts, 1♂, 2♀, 2 juveniles (WAM T118983); end of Mutton Bird Road, 35°02'52"S, 117°41'42"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1♂, 3 juveniles (WAM T89582DNA: MB-21-J); end of Mutton Bird Road, 35°02'40"S, 117°41'50"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1♀ (WAM T89581DNA: MB-20-F); end of Mutton Bird Road, 35°02'54"S, 117°42'07"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1♀ (WAM T89584DNA: MB-23-F); end of Mutton Bird Road, 35°02'42"S, 117°41'36"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89579); end of Mutton Bird Road, 35°02'43"S, 117°41'43"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89580); end of Mutton Bird Road, 35°02'58"S, 117°41'56"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89583). Porongurup National Park: west of Waddy's Hut, 34°40'54"S, 117°50'48"E, sifting elevated leaf litter in unburnt Karri forest, 306 m, 20.IV.2009, M. Rix, 1♀, 1 juvenile (WAM T97465DNA: PO-166-F/PO-167-J); same data except 6 March 2010, M. Rix, L. Lopardo, 1♀ (WAM T114028DNA: PO-168-F). Torndirrup Peninsula: Albany Wind Farm, 35°03'53"S, 117°47'35"E, beating vegetation in coastal heathland, 26.IX.2007, M. Harvey, R. Ott, 3 juveniles (WAM T89557); Albany Wind Farm, off Bibbulmun Track boardwalk, 35°03'57"S, 117°47'43"E, sifting elevated leaf litter under Banksia praemorsa, 12.III.2007, M. Rix, M. Harvey, M. Moir, 1 juvenile (WAM T81097); Albany Wind Farm, NW. of Wind Turbine No. 2, 35°03'43"S, 117°47'46"E, sifting elevated leaf litter under Agonis, 15.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89575DNA: WF-12-J); Albany Wind Farm, NW. of Wind Turbine No. 3, 35°03'44"S, 117°47'38"E, sifting elevated leaf litter under Agonis, 15.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89576DNA: WF-13-J); Albany Wind Farm, SW. of Wind Turbine No. 5, 35°03'35"S, 117°47'22"E, sifting elevated leaf litter under Agonis, 15.III.2008, M. Rix, M. Harvey, 1♀ (WAM T89574DNA: WF-11-F); along Bibbulmun Track E. of Albany Wind Farm, 35°04'05"S, 117°48'01"E, sifting elevated leaf litter under Agonis, 27.V.2011, M. Rix, M. Harvey, G. Binford, 1♂ (WAM T118982); along Bibbulmun Track E. of Albany Wind Farm, 35°04'05"S, 117°48'07"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1♀ (WAM T89585DNA: WF-24-F); along Bibbulmun Track E. of Albany Wind Farm, 35°04'10"S, 117°48'26"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89588); along Bibbulmun Track E. of Albany Wind Farm, 35°04'08"S, 117°48'18"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89587DNA: WF-26-J); along Bibbulmun Track E. of Albany Wind Farm, 35°04'06"S, 117°48'09"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89586DNA: WF-25-J); Cuthbert, W. of Roberts Road, 35°01'59"S, 117°47'47"E, sifting elevated leaf litter under Agonis, 18.III.2008, M. Rix, M. Harvey, 1♀, 2 juveniles (WAM T89589DNA: WF-28-J/WF-29-J); end of Prescott Vale Road, W. of Albany Wind Farm, 35°02'57"S, 117°45'30"E, sifting elevated leaf litter under Agonis, 15.III.2008, M. Rix, M. Harvey, 1♂, 3 juveniles (WAM T89569DNA: WF-5-J/WF-6-J); end of Prescott Vale Road, W. of Albany Wind Farm, 35°03'10"S, 117°46'11"E, sifting elevated leaf litter under Agonis, 15.III.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89573DNA: WF-10-J); end of Prescott Vale Road, W. of Albany Wind Farm, 35°03'10"S, 117°45'59"E, sifting elevated leaf litter under Agonis, 15.III.2008, M. Rix, M. Harvey, 1♀ (WAM T89572DNA: WF-9-F); end of Prescott Vale Road, W. of Albany Wind Farm, 35°03'02"S, 117°45'28"E, sifting elevated leaf litter under Agonis, 15.III.2008, M. Rix, M. Harvey, 1♀ (WAM T89570); end of Prescott Vale Road, W. of Albany Wind Farm, 35°03'10"S, 117°45'43"E, sifting elevated leaf litter under Agonis, 15.III.2008, M. Rix, M. Harvey, 1♀ (WAM T89571DNA: WF-8-F). Walpole-Nornalup National Park: Anderson Road, 34°59'43"S, 116°52'14"E, sifting elevated leaf litter under curly grass in Marri and Agonis forest, 3.V.2008, M. Rix, M. Harvey, 2 juveniles (WAM T89564DNA: WA-36-J/WA-37-J); same data except 24.I.2010, M. Rix, J. Wojcieszek, 1♂, 2♀, 1 juvenile (WAM T114037DNA: WA-162-F). William Bay National Park: near Elephant Rock carpark, 35°01'21"S, 117°14'12"E, sifting elevated leaf litter under Agonis, 26.VI.2010, M. Rix, 2♀ (WAM T114029DNA: WB-169-F); same data except 29.IV.2008, M. Rix, M. Harvey, 1 juvenile (WAM T89591DNA: WB-31-J); same data except 7.III.2010, M. Rix, L. Lopardo, 2 juveniles (WAM T114030DNA: WB-170-J).
Zephyrarchaea mainae can be distinguished from other known congeners except Zephyrarchaea janineae sp. n. by the presence of six dorsal, hump-like tubercles on the abdomen (Figs 1E–F, 6A, 10A–B); and from Zephyrarchaea janineae sp. n. by the more flattened, rounded apex of tegular sclerite 1 (TS 1) (Figs 10D–F).
This species can also be distinguished from other genotyped taxa (see Fig. 3) by the following two unique nucleotide substitutions for COI and COII (n = 35): A(282), T(1173).
Holotype male: Total length 3.44; leg I femur 1.81; F1/CL ratio 1.72. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige, with reddish-brown dorsal scute and sclerites (Fig. 10B). Carapace short (CH/CL ratio 1.60); 1.05 long, 1.68 high, 1.03 wide; ‘neck’ 0.60 wide; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.67), carapace with pronounced concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.24) (Fig. 8G). Chelicerae with proximal tuft and additional comb of accessory setae on anterior face of paturon (Figs 5A, 5E, 10C). Abdomen 1.69 long, 1.18 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Pedipalp fully expanded (see Platnick 1991b, figs 4–6). Unexpanded pedipalp (of WAM T89569) (Figs 10D–F) pyriform, with broad, distally curved embolus supported by conductor sclerites 1–2; tegular sclerite 1 (TS 1) porrect, slightly curved in prolateral view, with flattened, rounded apex; TS 2–3 projecting beyond retro-distal rim of tegulum.
Female (WAM T118983): Total length 3.31; leg I femur 1.97; F1/CL ratio 1.73. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 10A). Carapace short (CH/CL ratio 1.71); 1.14 long, 1.95 high, 1.10 wide; ‘neck’ 0.68 wide; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.63), carapace with pronounced concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.24) (Fig. 9E). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.00 long, 1.36 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia (Fig. 10G) with cluster of ≤ 15 sausage-shaped spermathecae either side of gonopore, clusters widely separated along midline of genital plate.
Variation: Males (n = 8): total length 2.82–3.44; carapace length 1.02–1.15; carapace height 1.62–1.80; CH/CL ratio 1.56–1.64. Females (n = 23): total length 2.77–3.82; carapace length 0.94–1.21; carapace height 1.45–1.99; CH/CL ratio 1.55–1.71. Specimens of Zephyrarchaea mainae from the eastern-most Bremer Bay population have significantly smaller hump-like tubercles on the abdomen compared to populations further west (see Fig. 1F cf. Figs 1E, 10A), and males from this Bremer Bay population also have a more distally paddle-shaped TS 1 morphology similar to Zephyrarchaea marki sp. n. from Cape Le Grand National Park. Rix and Harvey (2012) showed that the Bremer Bay population of Zephyrarchaea mainae is genetically distinct (Fig. 3), with no evidence of recent gene flow, indicating possible incipient speciation.
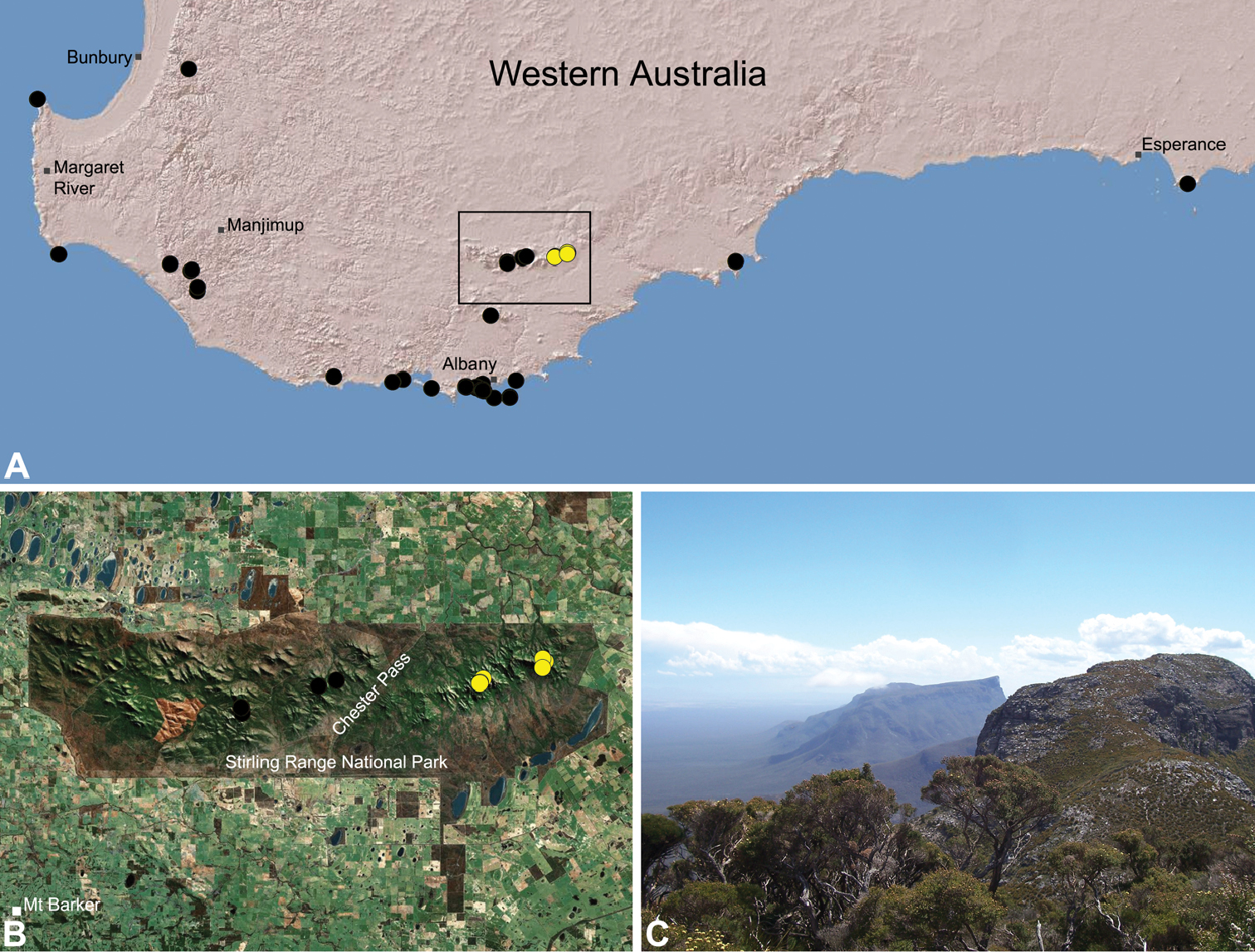
Zephyrarchaea mainae is known from the greater Albany region of southern Western Australia, from Walpole-Nornalup National Park (near Walpole) east to Bremer Bay and north to the Porongurup National Park, with a range centred on the Torndirrup Peninsula south of Albany (Fig. 20). Specimens have been collected by beating and sifting sedges (Lepidosperma sp.), curly grass (Empodisma gracillimum) and low shrubs in dense coastal or near-coastal groves of Peppermint (Agonis sp.), with several outlying populations also known from wet Karri (Eucalyptus diversicolor) forest.
Thisspecies is listed as threatened under theWestern Australian Wildlife Conservation Act 1950. It is a short-range endemic taxon (Harvey 2002b), with known populations threatened by fire, dieback disease (affecting coastal heathland vegetation), land-clearing and climate change.
urn:lsid:zoobank.org:act:3E20D50E-50A2-4D59-A2CD-565E05D4554D
http://species-id.net/wiki/Zephyrarchaea_janineae
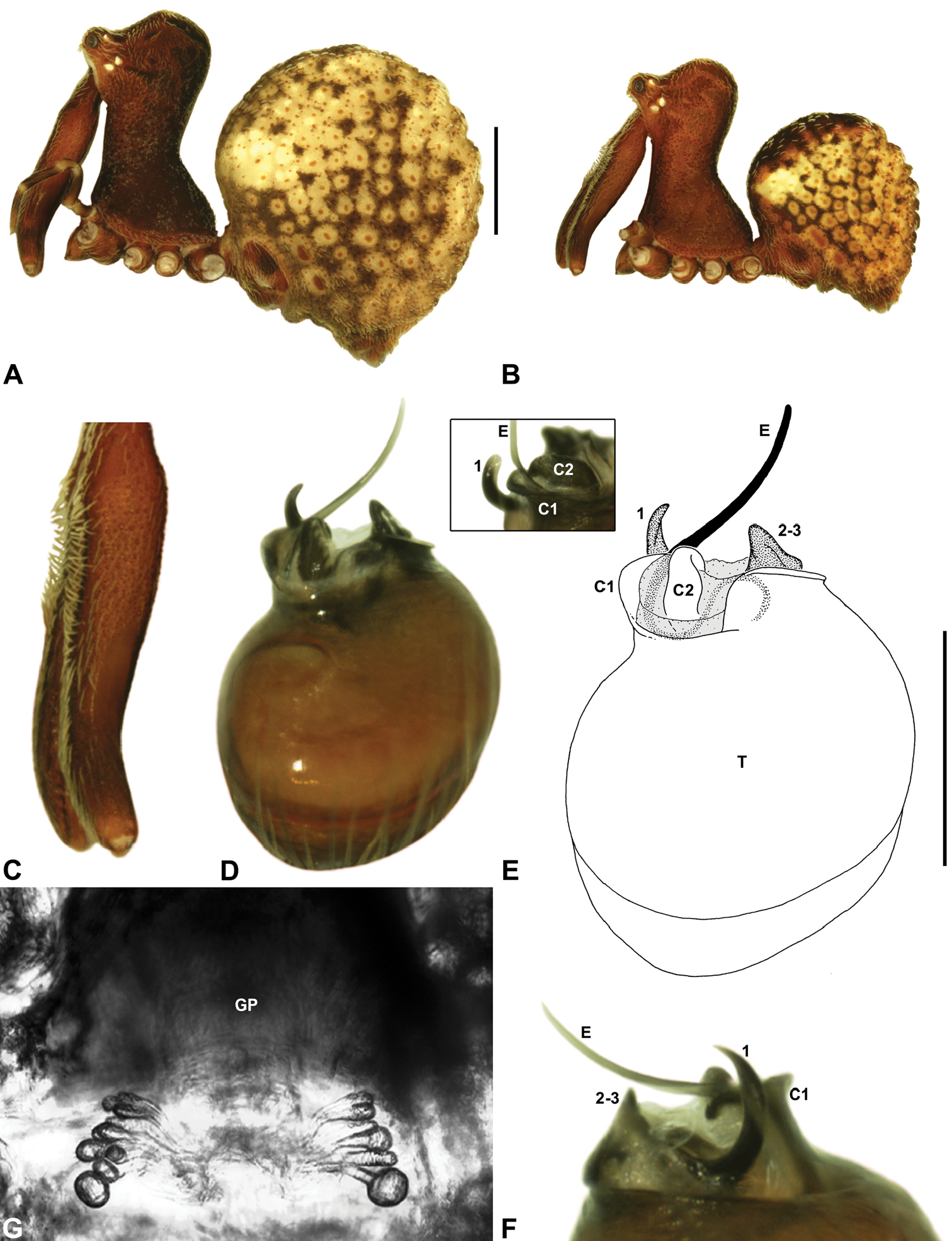
Karri Forest Assassin SpiderFigs 1C, 8I, 9F, 11, 21
Holotype male: Karri Valley, ‘Karri Valley Hideaway Cottages’, off Hopgarden Road, west of Pemberton, Western Australia, Australia, 34°24'59"S, 115°50'52"E, sifting elevated leaf litter in wet Marri and Agonis forest, 26–28.VIII.2006, M. Rix, J. Wojcieszek (WAM T89559).
Paratypes: Allotype female, same data as holotype (WAM T118981).
AUSTRALIA: Western Australia: Karri Valley: ‘Karri Valley Hideaway Cottages’, off Hopgarden Road, west of Pemberton, 34°24'57"S, 115°50'50"E, sifting elevated leaf litter in wet Marri and Agonis forest, 3.V.2008, M. Rix, M. Harvey, 2 juveniles (WAM T89565DNA: KV-38-J/KV-39-J). Greater Hawke National Park: Gloucester Road, ~360 m off Pemberton-Northcliffe Road, 34°21'01"S, 116°01'14"E, sifting leaf litter and beating grass-trees in very wet area, 16.X.2009, D. & S. Harms, 1 juvenile (WAM T114035DNA: GL-160-J). Dombakup State Forest: Marri Road, 15.I.1979, M. Gray, 4 juveniles (AMS KS15242). Leeuwin-Naturaliste National Park: near Cape Leeuwin, 34°22'00"S, 115°09'16"E, sifting elevated leaf litter in coastal Agonis forest, 15.VII.2009, M. Rix, 2♂ (WAM T94476); same data except 16.IV.2009, 1♀, 5 juveniles (WAM T97464DNA: CL-163-J/CL-164-J/CL-165-J); Sugarloaf Road, near Cape Naturaliste, 33°33'31"S, 115°01'18"E, sifting elevated leaf litter under Agonis, 30.III.2008, M. Rix, 1 juvenile (WAM T89561DNA: CN-32-J); Sugarloaf Road, near Cape Naturaliste, 33°33'29"S, 115°01'25"E, sifting elevated leaf litter under Agonis, 25.IV.2008, M. Rix, 2 juveniles (WAM T89562DNA: CN-33-J). Treen Brook State Forest: side road fire track off Vasse Highway, 34°26'45"S, 115°59'00"E, sifting leaf litter and teasing low shrubs, 14.X.2009, D. & S. Harms, 1 juvenile (WAM T114036DNA: TB-161-J); 8 km W. of Pemberton, 13.II.1979, M. Gray, 2 juveniles (AMS KS15341). Wellington National Park: Lennard Drive, near turnoff to Rapids Picnic Ground, 33°23’59”S, 115°57’52”E, sifting elevated leaf litter, dense Jarrah forest with Agonis on slope leading to Collie River, 141 m, 18.IX.2010, S. & D. Harms, 1♀ (WAM T112584DNA: CO-158-F); same data except 25.IX.2010, M. Rix, J. Wojcieszek, 1 juvenile (WAM T114034DNA: CO-159-J).
The specific epithet is a patronym in honour of Dr Janine Wojcieszek, for helping to discover the first live specimens of this species in 2006, and therefore catalysing the Western Australian Museum’s ‘archaeid project’ in the half decade since 2007.
Zephyrarchaea janineae can be distinguished from other known congeners except Zephyrarchaea mainae by the presence of six dorsal, hump-like tubercles on the abdomen (Figs 1C, 11A–B); and from Zephyrarchaea mainae by the more tapered, spiniform apex of tegular sclerite 1 (TS 1) (Figs 11D–F).
This species can also be distinguished from other genotyped taxa (see Fig. 3) by the following three unique nucleotide substitutions for COI (n = 11): G(87), G(132), T(609).
Holotype male: Total length 2.92; leg I femur 1.87; F1/CL ratio 1.76. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige, with reddish-brown dorsal scute and sclerites (Fig. 11B). Carapace short (CH/CL ratio 1.60); 1.06 long, 1.71 high, 1.01 wide; ‘neck’ 0.59 wide; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.65), carapace with pronounced concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.22) (Fig. 8I). Chelicerae with proximal tuft and additional comb of accessory setae on anterior face of paturon (Fig. 11C). Abdomen 1.54 long, 1.08 wide; with three pairs of dorsal hump-like tubercles (HT 1–6); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3–6 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 11D–F) pyriform, with broad, distally curved embolus supported by conductor sclerites 1–2; tegular sclerite 1 (TS 1) porrect, slightly curved in prolateral view, with tapered, spiniform apex; TS 2–3 projecting beyond retro-distal rim of tegulum.
Allotype female: Total length 4.10; leg I femur 1.97; F1/CL ratio 1.66. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 11A). Carapace short (CH/CL ratio 1.70); 1.19 long, 2.03 high, 1.15 wide; ‘neck’ 0.69 wide; highest point of pars cephalica (HPC) approaching posterior quarter of ‘head’ (ratio of HPC to post-ocular length 0.70), carapace with pronounced concave depression anterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.26) (Fig. 9F). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.62 long, 2.15 wide; with three pairs of dorsal hump-like tubercles (HT 1–6). Internal genitalia (Fig. 11G) with cluster of ≤ 15 sausage-shaped spermathecae either side of gonopore, clusters widely separated along midline of genital plate.
Variation: Males (n = 3): total length 2.92–3.15; carapace length 1.03–1.10; carapace height 1.67–1.82; CH/CL ratio 1.60–1.66. Females (n = 3): total length 3.03–4.10; carapace length 1.14–1.19; carapace height 1.92–2.03; CH/CL ratio 1.69–1.70.
Zephyrarchaea janineae is known from the high rainfall province (see Hopper and Gioia 2004) of southern Western Australia, from the Leeuwin-Naturaliste and Wellington National Parks (near Bunbury) east to Pemberton (Fig. 21). It is the dominant assassin spider of the south-western Karri (Eucalyptus diversicolor) forest and surrounding areas, and has been collected by beating and sifting elevated leaf litter in wet forested habitats and in coastal groves of Peppermint (Agonis sp.). Six juvenile specimens first collected by M. Gray in 1979 in the Treen Brook and Dombakup State Forests near Pemberton (see Main 1995) almost certainly belong to this species.
This specieshas a relatively widespread distribution in several National Parks and State Forests, and is not considered to be of conservation concern.
urn:lsid:zoobank.org:act:8EF9792F-4867-40B9-A409-2318A03E33E8
http://species-id.net/wiki/Zephyrarchaea_marki
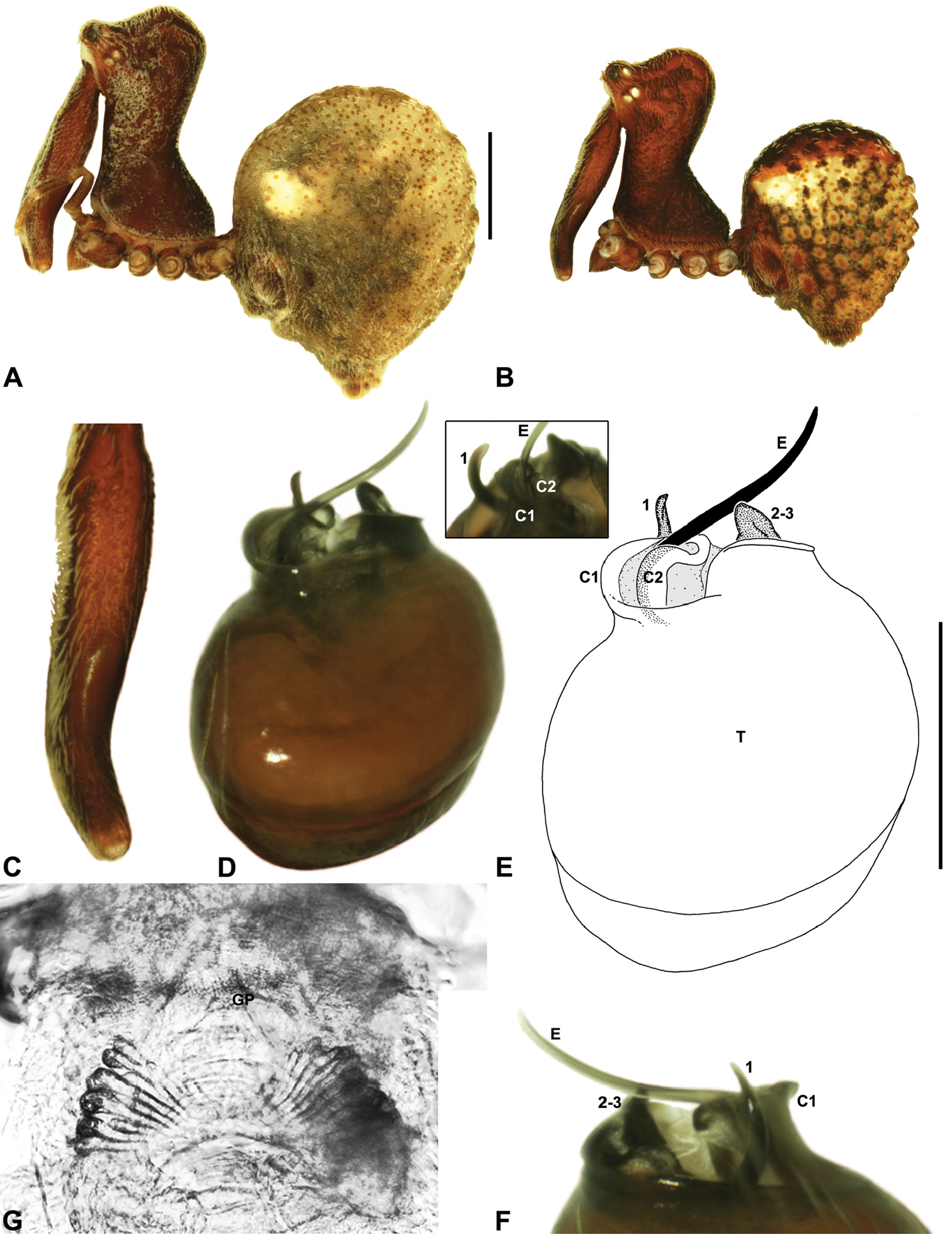
Cape Le Grand Assassin SpiderFigs 1D, 4B, 8H, 12, 22
Holotype male: Cape Le Grand National Park, Thistle Cove, Western Australia, Australia, 33°59'55"S, 122°11'59"E, sifting elevated leaf litter in Banksia speciosa thicket behind beach, 5.VI.2010, M. Rix (WAM T118985).
Paratypes: 1 male and 1 juvenile, same data as holotype (WAM T114033DNA: CLG-146-J).
AUSTRALIA: Western Australia: Cape Le Grand National Park: Thistle Cove, 33°59'54"S, 122°12'01"E, sifting elevated leaf litter, Banksia speciosa thicket behind beach, 28.VII.2009, M. Rix, M. Wojcieszek, 3 juveniles (WAM T94477DNA: CLG-144-J/CLG-145-J).
The specific epithet is a patronym in honour of Mark Wojcieszek, for helping to discover the first specimens of this species at Cape Le Grand National Park in 2009.
Zephyrarchaea marki can be distinguished from Zephyrarchaea janineae and Zephyrarchaea mainae by the absence of dorsal hump-like tubercles on the abdomen (Fig. 12A); from Zephyrarchaea marae sp. n. and Zephyrarchaea vichickmani sp. n. by the presence of a proximal tuft of accessory setae on the male chelicerae (Fig. 12B); from Zephyrarchaea barrettae sp. n. and Zephyrarchaea melindae sp. n. by the shape of tegular sclerites 2–3, which project well beyond the retro-distal rim of the tegulum (Figs 12C–D); and from Zephyrarchaea porchi sp. n. by the larger, more protuberant proximal bulge on the male chelicerae (Fig. 12B).
This species can also be distinguished from other genotyped taxa (see Fig. 3) by the following nine unique nucleotide substitutions for COI and COII (n = 3): A(147), T(204), C(300), C(306), G(495), C(804), G(807), G(1491), A(1548).
Holotype male: Total length 2.77; leg I femur 2.00; F1/CL ratio 1.95. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige, with reddish-brown dorsal scute and sclerites (Fig. 12A). Carapace short (CH/CL ratio 1.65); 1.03 long, 1.69 high, 1.00 wide; ‘neck’ 0.56 wide; highest point of pars cephalica (HPC) approaching posterior third of ‘head’ (ratio of HPC to post-ocular length 0.62), carapace with pronounced concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.21) (Fig. 8H). Chelicerae with proximal tuft and additional comb of accessory setae on anterior face of paturon (Fig. 12B). Abdomen 1.64 long, 1.13 wide; almost spherical in lateral profile, without dorsal hump-like tubercles but with highly recumbent mound-like vestiges; dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to cover nearly anterior two-thirds of dorsal abdomen. Unexpanded pedipalp (Figs 12C–E) pyriform, with broad, distally curved embolus supported by conductor sclerites 1–2; tegular sclerite 1 (TS 1) porrect, strongly curved in prolateral view, with flattened, broadly rounded, paddle-shaped apex; TS 2–3 projecting beyond retro-distal rim of tegulum.
Female: Unknown.
Variation: Males (n = 2): total length 2.77–2.79; carapace length 1.03 (invariable); carapace height 1.69 (invariable); CH/CL ratio 1.64–1.65.
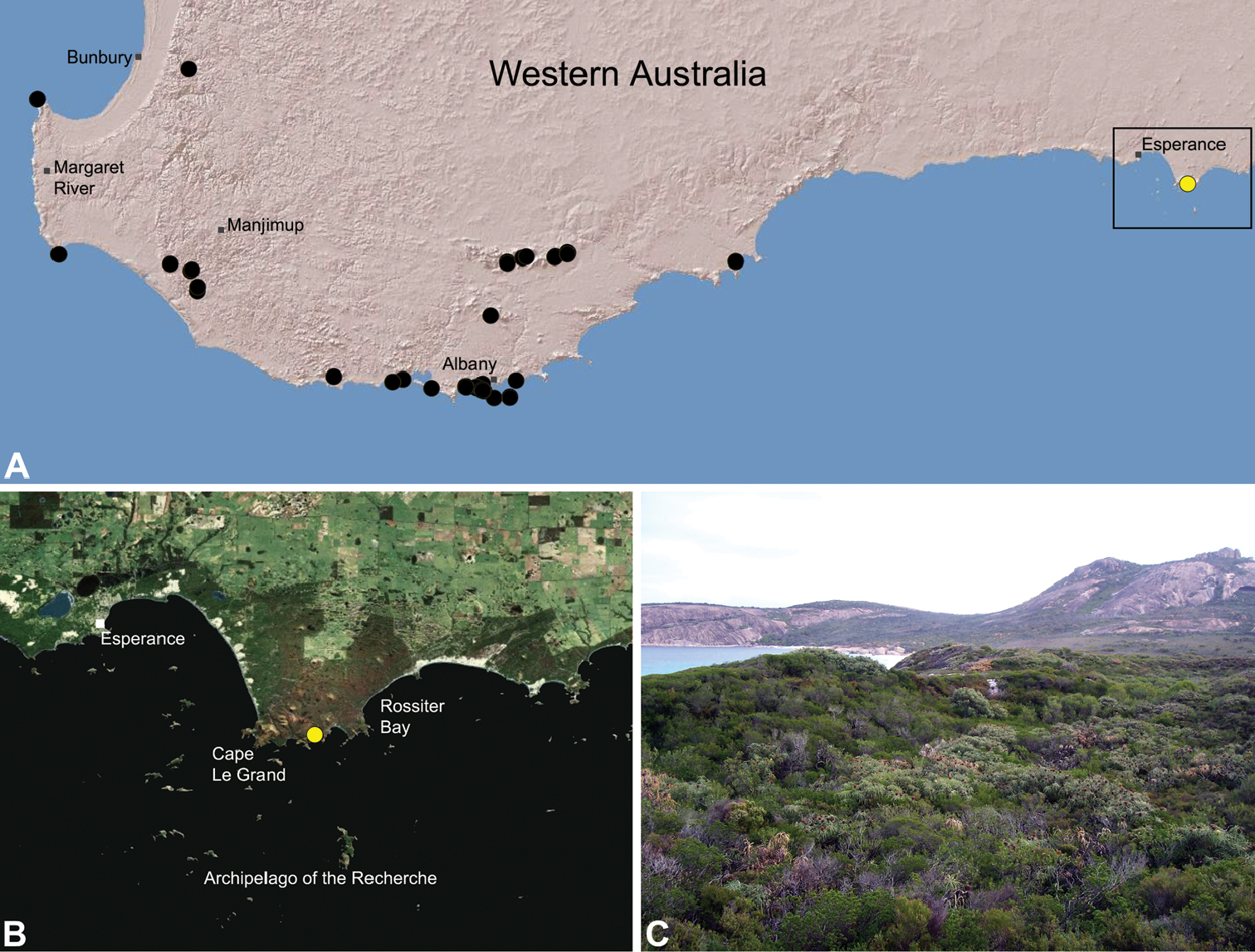
Zephyrarchaea marki is known only from Thistle Cove at Cape Le Grand National Park, on the far south-eastern coast of Western Australia (Fig. 22). Specimens have been collected by beating and sifting elevated leaf litter in a dense coastal thicket of Banksia speciosa.
This species appears to be a rare short-range endemic taxon (Harvey 2002b), with the single known population in the Cape Le Grand National Park potentially threatened by fire, dieback disease (affecting Banksia heathland vegetation) and climate change.
http://species-id.net/wiki/Zephyrarchaea_robinsi
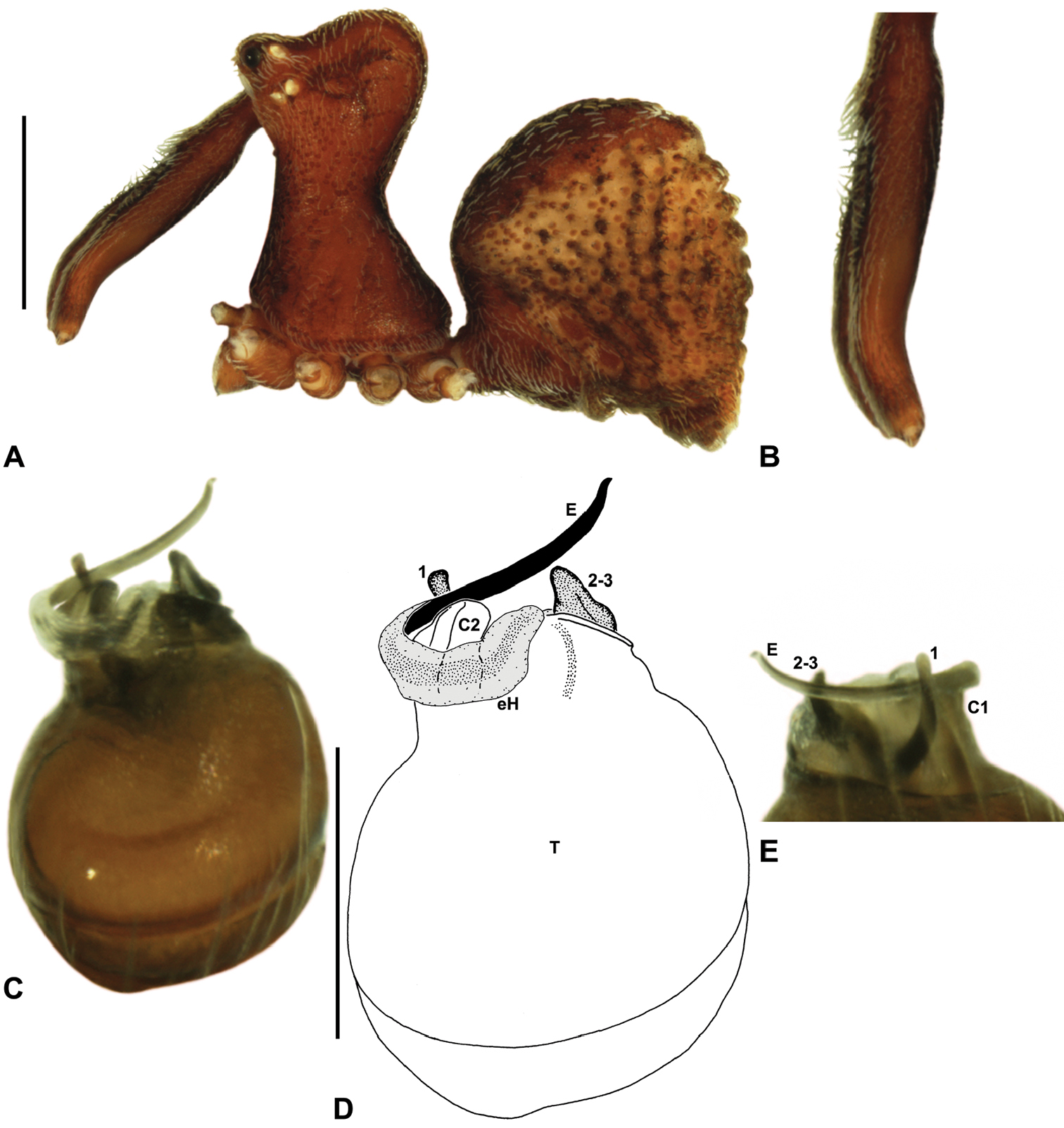
Eastern Massif Assassin SpiderFigs 9G, 13, 23
Holotype female: Stirling Range National Park, Ellen Peak, Western Australia, Australia, 34°21'20"S, 118°19'45"E, pitfall trap near summit, 28.V.1996, S. Barrett (WAM T42580).
AUSTRALIA: Western Australia: Stirling Range National Park: Ellen Peak, 34°21'30"S, 118°19'57"E, sifting elevated leaf litter under Lepidosperma sedges in montane heathland near summit, 1007 m, 6.XI.2007, M. Rix et al., 2 juveniles (WAM T89558DNA: EP-40-J/EP-41-J); south face of Pyungoorup Peak, 34°21'54"S, 118°19'44"E, sifting elevated leaf litter under Lepidosperma sedges along shaded creek line near waterfall, 5.VIII.2008, M. Rix, M. Harvey, 1 juvenile (moulted cuticle) (WAM T94090); Bluff Knoll, summit track, 800 m SW. of summit, 34°22'49"S, 118°15'01"E, sifting elevated leaf litter under Lepidosperma sedges in montane heathland, 897 m, 20.VI.2010, M. Rix, J.D. Roberts, 1 juvenile (WAM T114032DNA: BK-149-J); Bluff Knoll, off summit track, 900 m SW. of summit, 34°22'52"S, 118°15'00"E, sifting elevated leaf litter in eucalypt grove near creek line, 877 m, 20.VI.2010, M. Rix, J.D. Roberts, 1 juvenile (WAM T114031DNA: BK-148-J); Bluff Knoll, off summit track, 400 m SW. of summit, 34°22'34"S, 118°15'15"E, sifting elevated leaf litter under Lepidosperma sedges in montane heathland, 1065 m, 24.V.2011, M. Rix, M. Harvey, G. Binford, 1 juvenile (WAM T118987).
Females of Zephyrarchaea robinsi can be distinguished from Zephyrarchaea janineae and Zephyrarchaea mainae by the absence of dorsal hump-like tubercles on the abdomen (Fig. 13A); from Zephyrarchaea austini sp. n., Zephyrarchaea grayi sp. n., Zephyrarchaea marae sp. n. and Zephyrarchaea vichickmani sp. n. by the shallow post-ocular depression in lateral view (Fig. 9G); and from Zephyrarchaea barrettae sp. n. and Zephyrarchaea melindae sp. n. by the much shorter carapace (CH/CL ratio < 1.70) (Figs 7, 13A).
This species can also be distinguished from other genotyped taxa (see Fig. 3) by the following three unique nucleotide substitutions for COI and COII (n = 4): C(162), A(531), G(1442).
Holotype female: Total length 3.69; leg I femur 1.97; F1/CL ratio 1.60. Cephalothorax reddish-brown; legs tan brown with darker annulations; abdomen variably beige-grey (Fig. 13A). Carapace short (CH/CL ratio 1.60); 1.23 long, 1.97 high, 1.13 wide; ‘neck’ 0.74 wide; highest point of pars cephalica (HPC) approaching posterior third of ‘head’ (ratio of HPC to post-ocular length 0.61), carapace with shallow concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.24) (Fig. 9G). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.10 long, 1.78 wide; spherical in lateral profile, without dorsal hump-like tubercles. Internal genitalia (Figs 13B–D) with cluster of ≤ 15 sausage-shaped spermathecae either side of gonopore, clusters widely separated along midline of genital plate.
Male: Unknown.
Zephyrarchaea robinsi is known only from Ellen Peak, Bluff Knoll and the south face of Pyungoorup Peak, on the eastern massif of the Stirling Range National Park of southern Western Australia (east of Chester Pass) (Fig. 23). Specimens have been collected by beating and sifting sedges (Lepidosperma sp.) in montane heathland habitats and along mesic, shaded creek lines.
This species is a short-range endemic taxon (Harvey 2002b), with a maximum total range of less than 10 km2, and all known populations in the eastern Stirling Range National Park potentially threatened by fire, dieback disease (affecting montane vegetation) and climate change.
urn:lsid:zoobank.org:act:E42EAC7F-09B8-4441-85DD-4D84F44D923C
http://species-id.net/wiki/Zephyrarchaea_melindae
Toolbrunup Assassin SpiderFigs 8E, 9H, 14, 24
Holotype male: Stirling Range National Park, Mount Hassell, Western Australia, Australia, 34°22'41"S, 118°04'15"E, sifting elevated leaf litter under Lepidosperma sedges near summit, 726 m, 22.IV.2009, M. Rix (WAM T118986).
Paratypes: Allotype female and 1 juvenile, Toolbrunup Peak, 34°23'02"S, 118°02'55"E, sifting elevated leaf litter under low herbaceous shrubs near summit, 964 m, 10.IV.2009, M. Rix, H. Wood (WAM T97468DNA: TP-152-F/TP-153-J).
AUSTRALIA: Western Australia: Stirling Range National Park: same data as holotype, 2 juveniles (WAM T97467DNA: HA-150-J/HA-151-J).
The specific epithet is a patronym in honour of Dr Melinda Moir, in recognition of her contributions to biodiversity research, especially in the Stirling Range National Park of southern Western Australia.
Zephyrarchaea melindae can be distinguished from Zephyrarchaea janineae and Zephyrarchaea mainae by the absence of dorsal hump-like tubercles on the abdomen (Figs 14A–B); from Zephyrarchaea marae sp. n., Zephyrarchaea marki, Zephyrarchaea porchi sp. n. and Zephyrarchaea vichickmani sp. n. by the shape of tegular sclerites 2–3, which do not project beyond the retro-distal rim of the tegulum (Figs 14D–E); and from Zephyrarchaea barrettae sp. n. by the shape of the anterior margin of the diastema adjacent to the ‘neck’, which is straight (in females) or only slightly concave in lateral view (in males) (Figs 14A–B cf. Figs 15A–B). Females further distinguished from other known congeners by the combination of a spherical abdomen (Fig. 14A), shallow post-ocular depression in lateral view (Fig. 9H), taller carapace (CH/CL ratio > 1.70) (Figs 7, 14A) and straight, almost vertical anterior margin of the diastema adjacent to the ‘neck’ (Fig. 14A).
This species can also be distinguished from other genotyped taxa (see Fig. 3) by the following three unique nucleotide substitutions for COI and COII (n = 4): G(165), G(924), T(1533).
Holotype male: Total length 3.15; leg I femur 2.37; F1/CL ratio 2.01. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige, with reddish-brown dorsal scute and sclerites (Fig. 14B). Carapace relatively short (CH/CL ratio 1.77); 1.18 long, 2.09 high, 1.18 wide; ‘neck’ 0.68 wide; highest point of pars cephalica (HPC) approaching posterior third of ‘head’ (ratio of HPC to post-ocular length 0.62), carapace with shallow concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.20) (Fig. 8E). Chelicerae with proximal brush and additional comb of accessory setae on anterior face of paturon (Fig. 14C). Abdomen 1.64 long, 1.23 wide; almost spherical in lateral profile, without dorsal hump-like tubercles; dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to cover anterior two-thirds of dorsal abdomen. Unexpanded pedipalp (Figs 14D–F) pyriform, with broad, distally curved embolus supported by conductor sclerites 1–2; tegular sclerite 1 (TS 1) strongly curved, claw-like in prolateral view, with twisted, flattened and broadly rounded apex; TS 2–3 not projecting beyond retro-distal rim of tegulum.
Allotype female: Total length 3.54; leg I femur 2.50; F1/CL ratio 1.93. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 14A). Carapace relatively short (CH/CL ratio 1.86); 1.29 long, 2.41 high, 1.28 wide; ‘neck’ 0.78 wide; highest point of pars cephalica (HPC) approaching middle of ‘head’ (ratio of HPC to post-ocular length 0.57), carapace with shallow concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.19) (Fig. 9H). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.95 long, 1.51 wide; spherical in lateral profile, without dorsal hump-like tubercles. Internal genitalia (Fig. 14G) with cluster of ≤ 15 sausage-shaped spermathecae either side of gonopore, clusters widely separated along midline of genital plate.
Zephyrarchaea melindae is known only from the summits of Toolbrunup Peak and nearby Mount Hassell, in the western Stirling Range National Park of southern Western Australia (west of Chester Pass) (Fig. 24). Specimens have been collected by beating and sifting sedges (Lepidosperma sp.) and low shrubs in montane heathland habitats.
This species is a short-range endemic taxon (Harvey 2002b), with a maximum total range of less than 10 km2, and all known populations in the western Stirling Range National Park potentially threatened by fire, dieback disease (affecting montane vegetation) and climate change.
urn:lsid:zoobank.org:act:D01825F1-C02A-45C9-96F3-0329CA4F1308
http://species-id.net/wiki/Zephyrarchaea_barrettae
Talyuberlup Assassin SpiderFigs 1B, 8F, 9I, 15, 25
AUSTRALIA: Holotype male: Stirling Range National Park, Talyuberlup Peak, Western Australia, Australia, 34°24'21"S, 117°57'08"E, sifting elevated leaf litter under Lepidosperma sedges near summit, 4.VIII.2008, M. Rix, M. Harvey (WAM T117055DNA: TA-154-M).
Paratypes: Allotype female and 2 juveniles, same data as holotype except 8.II.2009, M. Harvey (WAM T97466DNA: TA-156-J/TA-157-J).
AUSTRALIA: Western Australia: Stirling Range National Park: same data as holotype, 1 juvenile (WAM T94089DNA: TA-155-J); Talyuberlup Peak, 34°24'20"S, 117°57'06"E, sifting elevated and low leaf litter, montane vegetation around rocky peak, 752 m, 12 April 2009, H. Wood, 1♂, 2♀ (CASENT 9028379); same data, 1♀ (CASENT 9034515).
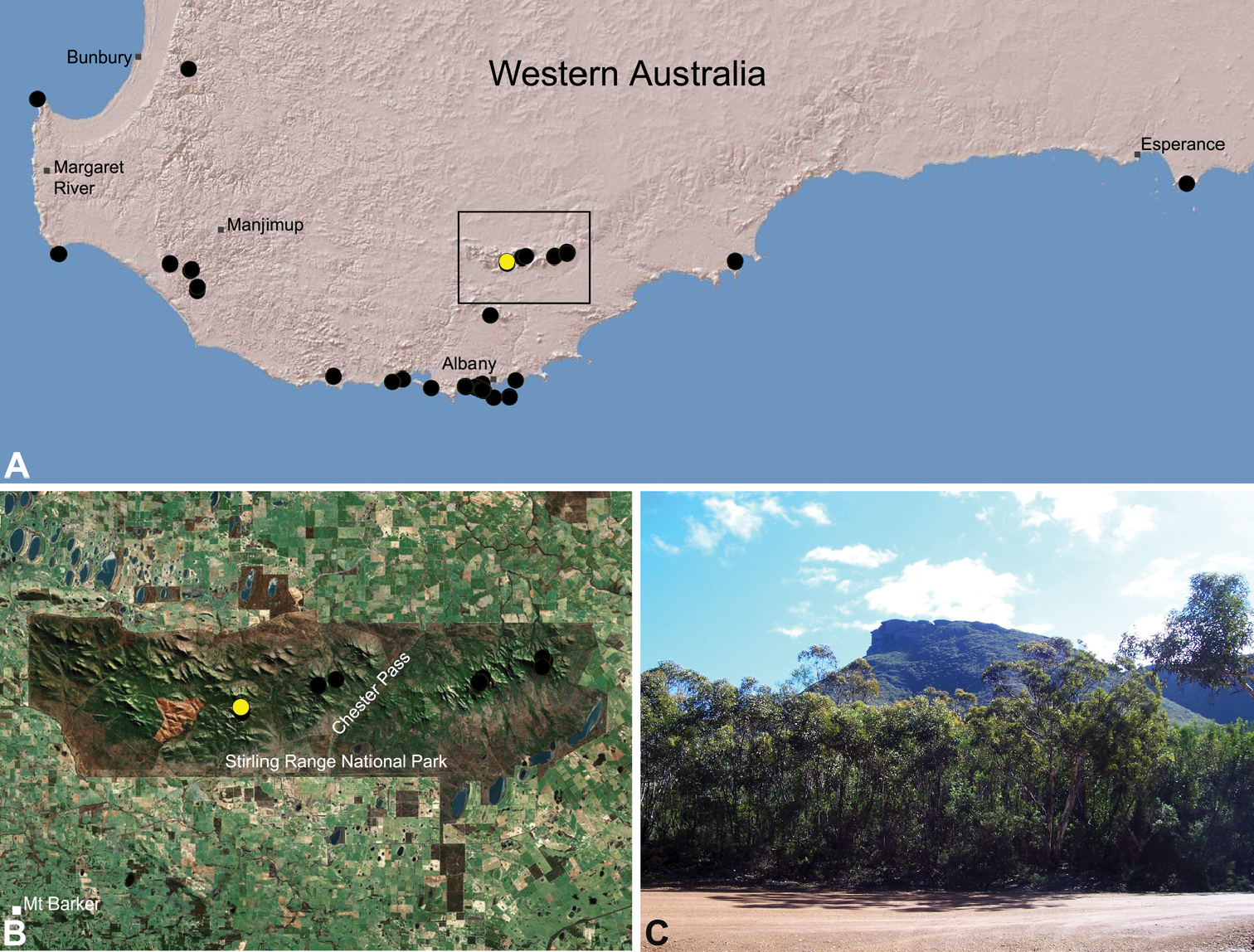
The specific epithet is a patronym in honour of Sarah Barrett, for first discovering assassin spiders in the Stirling Range National Park in 1996.
Zephyrarchaea barrettae can be distinguished from Zephyrarchaea janineae and Zephyrarchaea mainae by the absence of dorsal hump-like tubercles on the abdomen (Figs 15A–B); from Zephyrarchaea marae sp. n., Zephyrarchaea marki, Zephyrarchaea porchi sp. n. and Zephyrarchaea vichickmani sp. n. by the shape of tegular sclerites 2–3, which do not project beyond the retro-distal rim of the tegulum (Figs 15D–E); and from Zephyrarchaea melindae by the shape of the anterior margin of the diastema adjacent to the ‘neck’, which is slightly (in females) or strongly concave in lateral view (in males) (Figs 15A–B cf. Figs 14A–B). Females further distinguished from other known congeners by the combination of a spherical abdomen (Fig. 15A), shallow post-ocular depression in lateral view (Fig. 9I), taller carapace (CH/CL ratio > 1.70) (Figs 7, 15A) and slightly concave anterior margin of the diastema adjacent to the ‘neck’ (Fig. 15A).
Holotype male: Total length 3.13; leg I femur 2.19; F1/CL ratio 1.92. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige, with reddish-brown dorsal scute and sclerites (Fig. 15B). Carapace relatively short (CH/CL ratio 1.71); 1.14 long, 1.95 high, 1.13 wide; ‘neck’ 0.67 wide; highest point of pars cephalica (HPC) approaching posterior third of ‘head’ (ratio of HPC to post-ocular length 0.59), carapace with shallow concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.21) (Fig. 8F). Chelicerae with proximal brush and additional comb of accessory setae on anterior face of paturon (Fig. 15C). Abdomen 1.59 long, 1.21 wide; almost spherical in lateral profile, without dorsal hump-like tubercles; dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to cover anterior two-thirds of dorsal abdomen. Unexpanded pedipalp (Figs 15D–F) pyriform, with broad, distally curved embolus supported by conductor sclerites 1–2; tegular sclerite 1 (TS 1) strongly curved, claw-like in prolateral view, with twisted, flattened and broadly rounded apex; TS 2–3 not projecting beyond retro-distal rim of tegulum.
Allotype female: Total length 3.64; leg I femur 2.31; F1/CL ratio 1.77. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen variably beige-grey (Fig. 15A). Carapace relatively short (CH/CL ratio 1.78); 1.31 long, 2.33 high, 1.26 wide; ‘neck’ 0.78 wide; highest point of pars cephalica (HPC) approaching posterior third of ‘head’ (ratio of HPC to post-ocular length 0.59), carapace with shallow concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.22) (Fig. 9I). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.90 long, 1.69 wide; spherical in lateral profile, without dorsal hump-like tubercles. Internal genitalia (Fig. 15G) with cluster of ≤ 15 sausage-shaped spermathecae either side of gonopore, clusters widely separated along midline of genital plate.
Zephyrarchaea barrettae is known only from the summit of Talyuberlup Peak, in the western Stirling Range National Park of southern Western Australia (west of Chester Pass) (Fig. 25). Specimens have been collected by beating and sifting sedges (Lepidosperma sp.) in montane heathland.
This species is a short-range endemic taxon (Harvey 2002b), with a maximum total range of less than 10 km2, and all known populations in the western Stirling Range National Park potentially threatened by fire, dieback disease (affecting montane vegetation) and climate change.
In the absence of adult specimens or molecular data, the following juvenile specimens from Western Australia could not be confidently identified as a known species.
AUSTRALIA: Western Australia: Stirling Range National Park: Talyuberlup Picnic Area, gully 400 m NW. of carpark, 34°24'42"S, 117°57'12"E, sifting grass patches within gully, 21.VI.2011, D. & S. Harms, 4 juveniles (WAM T118991).
These specimens are the first Archaeidae to be collected from lowland habitats in the Stirling Range National Park, and the first members of the Western Australian High Rainfall Zone Clade (Fig. 3) to be discovered in the Stirling Range. All four juveniles possess paired dorsal tubercles on the abdomen, clearly aligning them with Zephyrarchaea mainae and Zephyrarchaea janineae. Zephyrarchaea mainae has a known distribution that extends north to the Porongurup National Park (see Fig. 20), and it is possible that the Talyuberlup Picnic Area may represent a northern extension of this range. Adult specimens or sequence data are required to confirm the identification of this population.
Holotype juvenile (not examined): no specific locality, Victoria, Australia, ~1922 (MV K097).
AUSTRALIA: Victoria: no specific locality, 1936, C. Oke, 1 juvenile (AMS KS97261).
Archaea hickmani was first described by Butler (1929) from a juvenile specimen of unspecified providence, labelled and listed by Butler simply as “Victoria”. Forster and Platnick (1984) examined this holotype, stating that it was in rather poor condition, and noting that a second adult female (labelled as a “homotype”) accompanied the specimen, the latter apparently collected after the original description in 1929. The genitalia of this adult female specimen were illustrated in Forster and Platnick (1984, fig. 69), the specimen was briefly described, and the species was transferred to the genus Austrarchaea. Forster and Platnick (1984, figs 40–50) also presented scanning electron micrographs of a juvenile archaeid from near Sydney, erroneously regarded as being conspecific or very closely related to Austrarchaea hickmani based on the absence of setose tubercles on the carapace. However, this specimen is clearly a juvenile of an unrelated species of Austrarchaea, as evidenced by the abdominal tubercles (see Forster and Platnick 1984, fig. 40) and New South Wales distribution. The Australian Museum collection also has an additional juvenile specimen of Austrarchaea hickmani collected by C. Oke in 1936, similarly labelled as being from “Victoria”.
Based on the three known Victorian specimens identified by Butler as Archaea hickmani, the species is clearly congeneric and probably even conspecific with one of the four new Victorian species of Zephyrarchaea described in this paper. Unfortunately, given the unspecified collection locality of all three specimens, and thus the inability to unequivocally link the single adult female to the holotype or the type locality, this species must be regarded as a nomen dubium.
urn:lsid:zoobank.org:act:ADCE3562-8F83-49BE-BB5F-F794E7FECB41
http://species-id.net/wiki/Zephyrarchaea_vichickmani
Central Highlands Assassin SpiderFigs 1A, 8B, 9A, 16, 26
Holotype male: Yarra Ranges National Park, Acheron Gap, Victoria, Australia, 37°40'37"S, 145°44'24"E, sifting elevated leaf litter under tree ferns, Nothofagus rainforest, 769 m, 29.III.2010, M. Rix (MV K11578).
Paratypes: Allotype female, same data as holotype (MV K11579); 1 female and 6 juveniles, same data as holotype (WAM T112583DNA: Ar14–49-F/Ar14–133-J/Ar14–134-J).
AUSTRALIA: Victoria: Yarra Ranges National Park: Acheron Gap, Central Highlands, 6 km NE. of Mount Donna Buang, 37°40'43"S, 145°44'20"E, pitfall trap, Nothofagus cunninghamii forest, 25.VI.–29.VIII.1996, G. Milledge, 1♀ (MV K5919); same data except 21.II.–23.IV.1996, 1 juvenile (MV K5920).
The specific epithet is a patronym in honour of the late Professor Victor Hickman, for his extraordinary contributions to arachnology and in honour of L. S. Butler’s original (1929) patronym.
Zephyrarchaea vichickmani can be distinguished from other known congeners except Zephyrarchaea marae sp. n. by the absence of a proximal tuft or brush on the male chelicerae (Fig. 16C); and from Zephyrarchaea marae sp. n. by the less sinuous shape of conductor sclerite 2 (C2) (Figs 16D–E) and the more concave anterior margin of the male diastema adjacent to the ‘neck’ (Fig. 16B). Females further distinguished from other known congeners except Zephyrarchaea marae sp. n. by the combination of a spherical abdomen (Fig. 16A), strongly concave post-ocular depression in lateral view (Fig. 9A), and moderately elevated ‘head’ dorsally (post-ocular ratio ≥ 0.25) (Fig. 9A).
This species can also be distinguished from other genotyped taxa (see Fig. 3) by the following 16 unique nucleotide substitutions for COI and COII (n = 3): C(24), G(54), G(216), G(309), A(360), A(393), C(702), G(795), T(951), A(976), A(1059), T(1063), A(1200), G(1281), C(1479), T(1596), and can be further distinguished from all other Australian Archaeidae except Zephyrarchaea marae by the absence of a COII amino acid residue at positions 1441–1443.
Holotype male: Total length 2.77; leg I femur 1.80; F1/CL ratio 1.67. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige, with reddish-brown dorsal scute and sclerites (Fig. 16B). Carapace relatively short (CH/CL ratio 1.69); 1.08 long, 1.82 high, 1.01 wide; ‘neck’ 0.59 wide; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.64), carapace with pronounced concave depression anterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.28) (Fig. 8B). Chelicerae with short comb of accessory setae on anterior face of paturon (Fig. 16C). Abdomen 1.59 long, 1.10 wide; almost spherical in lateral profile, without dorsal hump-like tubercles; dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to cover anterior two-thirds of dorsal abdomen. Unexpanded pedipalp (Figs 16D–F) bulbous, almost spherical, with gently curved, tapering embolus supported by conductor sclerites 1–2; tegular sclerite 1 (TS 1) strongly curved, claw-like in prolateral view, with twisted, flattened and rounded apex; TS 2–3 projecting well beyond retro-distal rim of tegulum.
Allotype female: Total length 3.90; leg I femur 2.08; F1/CL ratio 1.76. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 16A). Carapace relatively short (CH/CL ratio 1.83); 1.18 long, 2.15 high, 1.12 wide; ‘neck’ 0.69 wide; highest point of pars cephalica (HPC) approaching posterior third of ‘head’ (ratio of HPC to post-ocular length 0.61), carapace with pronounced concave depression anterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.26) (Fig. 9A). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.51 long, 1.92 wide; spherical in lateral profile, without dorsal hump-like tubercles. Internal genitalia (Fig. 16G) with cluster of ≤ 15 sausage-shaped spermathecae fanning out either side of gonopore, clusters widely separated along midline of genital plate; outermost (posterior) spermatheca on each side stalked, distally spherical.
Variation: Females (n = 3): total length 3.44–3.90; carapace length 1.14–1.18; carapace height 2.00–2.15; CH/CL ratio 1.75–1.83.
Zephyrarchaea vichickmani is known only from temperate Nothofagus rainforest habitats in the Victorian Central Highlands, north-east of Melbourne (Fig. 26).
This species has an imperfectly known distribution, and although potentially restricted, the abundance of protected forested habitats near the type locality would suggest that the species is unlikely to be of conservation concern.
urn:lsid:zoobank.org:act:010F55AE-B08E-4725-8781-5157D69EDCBA
http://species-id.net/wiki/Zephyrarchaea_marae
West Gippsland Assassin SpiderFigs 4D, 5C–D, 5F–G, 6B, 6E, 8A, 8C, 9B, 17, 27
Holotype male: Tarra-Bulga National Park, Tarra Valley, near Tarra Valley Picnic Area, Victoria, Australia, 38°26'51"S, 146°32'17"E, sifting elevated leaf litter under tree ferns, Nothofagus rainforest, 368 m, 1.IV.2010, M. Rix, D. Harms (MV K11580DNA: Ar18–138-M).
Paratypes: Allotype female, Gunyah Rainforest State Reserve, Toorah Road, 2 km SSW. of Gunyah, Victoria, Australia, 38°32'30"S, 146°19'00"E, pitfall trap, Nothofagus cunninghamii forest, 14.IX.–14.XI.1995, G. Milledge (MV K5921); 1 male, same data except 5.III.–7.V.1996 (MV K5923).
AUSTRALIA: Victoria: Tarra-Bulga National Park: same data as holotype, 3 juveniles (WAM T114025DNA: Ar18–139-J/Ar18–140-J); 0.2 km W. of Tarra Valley Picnic Area, 38°27'S, 146°32'E, pitfall trap, Nothofagus cunninghamii forest, 14.XI.1995 – 10.I.1996, G. Milledge, 1 juvenile (MV K5922). Dandenong Ranges National Park: Sherbrooke Forest, near start of Welch Track, off Nation Road, 37°54'24"S, 145°22'10"E, sifting elevated leaf litter under tree ferns, wet Mountain Ash/tree fern forest, 28.III.2010, M. Rix, D. Harms, 1♀, 2 juveniles (WAM T114024DNA: Ar13–135-F/Ar13–136-J/Ar13–137-J). Mount Worth State Park: Giants Circuit from Moonlight Creek Picnic Area, 38°16'54"S, 146°00'35"E, sifting elevated leaf litter under tree fern, complex eucalypt/tree fern forest with thick understorey, 400 m, 31.III.2010, M. Rix, D. Harms, 1 juvenile (WAM T114026DNA: Ar16–141-J).
The specific epithet is a patronym in honour of Dr Māra Blosfelds, in recognition of her love for small spiders and the Australian forests.
Zephyrarchaea marae can be distinguished from other known congeners except Zephyrarchaea vichickmani by the absence of a proximal tuft or brush on the male chelicerae (Fig. 17C); and from Zephyrarchaea vichickmani by the more sinuous, slender, S-shaped conductor sclerite 2 (C2) (Figs 17D–E) and the less concave, slightly convex anterior margin of the male diastema adjacent to the ‘neck’ (Fig. 17B). Females further distinguished from other known congeners except Zephyrarchaea vichickmani by the combination of a spherical abdomen (Fig. 17A), strongly concave post-ocular depression in lateral view (Fig. 9B), and moderately elevated ‘head’ dorsally (post-ocular ratio ≥ 0.25) (Fig. 9B).
This species can also be distinguished from other genotyped taxa (see Fig. 3) by the following 12 unique nucleotide substitutions for COI and COII (n = 7): G(69), G(345), G(552), G(762), G(786), A(1026), G(1059), T(1263), G(1341), G(1512), C(1584), C(1587), and can be further distinguished from all other Australian Archaeidae except Zephyrarchaea vichickmani by the absence of a COII amino acid residue at positions 1441–1443.
Holotype male: Total length 3.03; leg I femur 1.99; F1/CL ratio 1.80. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige, with reddish-brown dorsal scute and sclerites (Fig. 17B). Carapace relatively short (CH/CL ratio 1.70); 1.10 long, 1.87 high, 1.08 wide; ‘neck’ 0.61 wide; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.66), carapace with pronounced concave depression anterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.25) (Figs 8A, 8C). Chelicerae with short comb of accessory setae on anterior face of paturon (Fig. 17C). Abdomen 1.64 long, 1.31 wide; almost spherical in lateral profile, without dorsal hump-like tubercles; dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to cover anterior two-thirds of dorsal abdomen. Unexpanded pedipalp (Figs 17D–F) bulbous, almost spherical, with gently curved, tapering embolus supported by conductor sclerites 1–2 (C1–2); C2 relatively slender, sinuous, with S-shaped proximal portion; tegular sclerite 1 (TS 1) strongly curved, claw-like in prolateral view, with twisted, flattened and rounded apex; TS 2–3 projecting well beyond retro-distal rim of tegulum.
Allotype female: Total length 3.95; leg I femur 2.10; F1/CL ratio 1.76. Cephalothorax dark reddish-brown (with paler, partially encrusted material on ‘neck’); legs tan brown with darker annulations; abdomen variably beige-grey (Fig. 17A). Carapace relatively short (CH/CL ratio 1.86); 1.19 long, 2.22 high, 1.17 wide; ‘neck’ 0.72 wide; highest point of pars cephalica (HPC) approaching posterior third of ‘head’ (ratio of HPC to post-ocular length 0.62), carapace with pronounced concave depression anterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.26) (Fig. 9B). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.31 long, 1.90 wide; spherical in lateral profile, without dorsal hump-like tubercles. Internal genitalia (Fig. 17G) with cluster of ≤ 15 sausage-shaped spermathecae fanning out either side of gonopore, clusters widely separated along midline of genital plate.
Variation: Males (n = 2): total length 3.03–3.23; carapace length 1.09–1.10; carapace height 1.85–1.87; CH/CL ratio 1.69–1.70. Females (n = 2): total length 3.74–3.95; carapace length 1.18–1.19; carapace height 2.09–2.22; CH/CL ratio 1.77–1.86.
Zephyrarchaea marae is known only from temperate rainforest and mesic closed forest habitats in the Dandenong and Strzelecki Ranges of West Gippsland, east and south-east of Melbourne, Victoria (Fig. 27).
This specieshas a relatively widespread distribution in several National Parks and State Forests, and is not considered to be of conservation concern.
urn:lsid:zoobank.org:act:3005E6AB-024D-4559-BAE7-78E3D47CE5E0
http://species-id.net/wiki/Zephyrarchaea_porchi
Otway Range Assassin SpiderFigs 8D, 18, 28
Holotype male: Bimbi Park, 2.2 km N. of Cape Otway Lighthouse, Victoria, Australia, 38°50'13"S, 143°30'55"E, dry pitfall trap, grassy edge of bracken-rich dry sclerophyll forest, 2–5.XI.2011, N. Porch & Deakin University Wildlife Field Studies students (MV K11581).
The specific epithet is a patronym in honour of Dr Nicholas Porch, for first discovering this species in the Otway Range.
Zephyrarchaea porchi can be distinguished from Zephyrarchaea janineae and Zephyrarchaea mainae by the absence of dorsal hump-like tubercles on the abdomen (Fig. 18A); from Zephyrarchaea marae and Zephyrarchaea vichickmani by the presence of a proximal tuft of accessory setae on the male chelicerae (Fig. 18B); from Zephyrarchaea barrettae and Zephyrarchaea melindae by the shape of tegular sclerites 2–3, which project well beyond the retro-distal rim of the tegulum (Figs 18C–D); and from Zephyrarchaea marki by the smaller, less protuberant proximal bulge on the male chelicerae (Fig. 18B).
Holotype male: Total length 2.77; leg I femur 1.97; F1/CL ratio 1.86. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige, with reddish-brown dorsal scute and sclerites (Fig. 18A). Carapace relatively short (CH/CL ratio 1.70); 1.06 long, 1.81 high, 1.00 wide; ‘neck’ 0.58 wide; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.69), carapace with pronounced concave depression anterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.27) (Fig. 8D). Chelicerae with proximal tuft and additional comb of accessory setae on anterior face of paturon (Fig. 18B). Abdomen 1.59 long, 1.08 wide; almost spherical in lateral profile, without dorsal hump-like tubercles; dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to cover anterior two-thirds of dorsal abdomen. Partially expanded pedipalp (Figs 18C–E) bulbous-pyriform, with gently curved, tapering embolus adjacent to conductor sclerites 1–2; tegular sclerite 1 (TS 1) porrect, slightly curved in prolateral view, with flattened, rounded apex; TS 2–3 projecting well beyond retro-distal rim of tegulum.
Female: Unknown.
Zephyrarchaea porchi is known only from north of Cape Otway, in the Otway Range of southern Victoria (Fig. 28). The single known specimen was collected in a dry vertebrate pitfall trap in eucalypt forest with a dense bracken fern understorey.
This species has an imperfectly known distribution, and although potentially restricted, the abundance of protected forested habitats near the type locality would suggest that the species is unlikely to be of conservation concern.
urn:lsid:zoobank.org:act:B449FA7D-B159-42D5-8F8F-74639C04575E
http://species-id.net/wiki/Zephyrarchaea_grayi
Grampians Assassin SpiderFigs 9C, 19A–B, 29
Holotype female: Grampians National Park, Delley’s Dell, Silverband Road, Victoria, Australia, sweeping at night, 26.III.1974, M. Gray (AMS KS109448).
The specific epithet is a patronym in honour of Dr Mike Gray, for his contributions to arachnology and for first discovering this species in the Grampians National Park.
Females of Zephyrarchaea grayi can be distinguished from Zephyrarchaea janineae and Zephyrarchaea mainae by the absence of dorsal hump-like tubercles on the abdomen (Fig. 19A); from Zephyrarchaea barrettae, Zephyrarchaea melindae and Zephyrarchaea robinsi by the strongly concave post-ocular depression in lateral view (Fig. 9C); from Zephyrarchaea austini sp. n. by the larger body size (carapace length > 1.10) and taller carapace (CH/CL ratio ≥ 1.70) (Figs 7, 19A); and from Zephyrarchaea marae and Zephyrarchaea vichickmani by the shape of the ‘head’, which is less elevated dorsally (post-ocular ratio < 0.25) and with the highest point of the pars cephalica (HPC) closer to the middle of the head (ratio of HPC to post-ocular length < 0.60) (Fig. 9C).
Holotype female: Total length 3.36; leg I femur 1.95; F1/CL ratio 1.73. Cephalothorax dark reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 19A). Carapace relatively short (CH/CL ratio 1.72); 1.13 long, 1.94 high, 1.03 wide; ‘neck’ 0.63 wide; highest point of pars cephalica (HPC) approaching middle of ‘head’ (ratio of HPC to post-ocular length 0.57), carapace with pronounced concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.23) (Fig. 9C). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.85 long, 1.36 wide; spherical in lateral profile, without dorsal hump-like tubercles. Internal genitalia (Fig. 19B) with cluster of ≤ 15 sausage-shaped spermathecae fanning out either side of gonopore, clusters widely separated along midline of genital plate; outermost (posterior) spermathecae bulbous distally.
Male: Unknown.
Zephyrarchaea grayi is known only from wet eucalypt forest at Delley’s Dell, in the Grampians National Park of western Victoria (Fig. 29).
This species appears to be a rare short-range endemic taxon (Harvey 2002b), with the single known population in the Grampians National Park potentially threatened by fire and climate change. Targeted searching at the type locality in March 2010 failed to reveal new specimens of this species, and the site had been recently burnt.
urn:lsid:zoobank.org:act:9571EBAF-A9E3-4053-9549-D36E06806DBE
http://species-id.net/wiki/Zephyrarchaea_austini
Kangaroo Island Assassin SpiderFigs 9D, 19C-D, 30
Holotype female: Kangaroo Island, Western River Wilderness Protection Area, Waterfall Creek walking trail, near waterfall, South Australia, Australia, 35°41'44"S, 136°54'37"E, sifting elevated leaf litter, open eucalypt woodland with complex understorey of Xanthorrhoea and low shrubs, 9-10.V.2010, M. Rix, D. Harms (SAM NN28000DNA: Ar77-50-F).
AUSTRALIA: South Australia: Western River Wilderness Protection Area: same data as holotype, 3 juveniles (WAM T114027DNA: Ar77-142-J/Ar77-143-J).
The specific epithet is a patronym in honour of Professor Andy Austin, in recognition of his contributions to biodiversity research.
Females of Zephyrarchaea austini can be distinguished from Zephyrarchaea janineae and Zephyrarchaea mainae by the absence of dorsal hump-like tubercles on the abdomen (Fig. 19C); from Zephyrarchaea barrettae, Zephyrarchaea melindae and Zephyrarchaea robinsi by the strongly concave post-ocular depression in lateral view (Fig. 9D); and from Zephyrarchaea grayi, Zephyrarchaea marae and Zephyrarchaea vichickmani by the smaller body size (carapace length < 1.10) and shorter carapace (CH/CL ratio < 1.70) (Figs 7, 19C).
This species can also be distinguished from other genotyped taxa (see Fig. 3) by the following 45 unique nucleotide substitutions for COI and COII (n = 3): T(42), A(45), C(130), T(132), G(222), A(243), G(291), A(354), G(360), T(372), A(453), A(504), G(567), G(648), A(690), A(702), C(717), C(721), G(756), C(852), A(858), A(891), G(901), A(910), G(962), C(971), G(1050), G(1165), G(1197), A(1198), T(1233), G(1234), C(1235), T(1236), G(1317), A(1374), A(1403), T(1404), G(1416), G(1418), A(1419), A(1449), T(1530), G(1574), G(1585).
Holotype female: Total length 2.77; leg I femur 1.80; F1/CL ratio 1.80. Cephalothorax pale reddish-brown; legs tan brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 19C). Carapace short (CH/CL ratio 1.58); 1.00 long, 1.58 high, 0.92 wide; ‘neck’ 0.55 wide; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.67), carapace with pronounced concave depression anterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.23) (Fig. 9D). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.49 long, 1.13 wide; almost spherical in lateral profile, without dorsal hump-like tubercles. Internal genitalia (Fig. 19D) with cluster of ≤ 15 sausage-shaped spermathecae fanning out either side of gonopore, clusters widely separated along midline of genital plate.
Male: Unknown.
Zephyrarchaea austini is known only from eucalypt woodland and associated heathland habitats near ‘Billy Goat Falls’, in the Western River Wilderness Protection Area of Kangaroo Island, South Australia (Fig. 30).
This species appears to be a rare short-range endemic taxon (Harvey 2002b), with the single known population on Kangaroo Island potentially threatened by fire, dieback disease (affecting heathland vegetation) and climate change.
This research was made possible by an Australian Biological Resources Study (ABRS) taxonomy research grant to M. Harvey and M. Rix (Grant No. 209-09), with critical initial support provided by Roy Teale (Biota Environmental Sciences) and significant additional funding provided by Cliffs Natural Resources and Verve Energy. Specimens of Zephyrarchaea were loaned by various curators, and the authors would like to especially thank Peter Lillywhite (Museum Victoria), Graham Milledge (Australian Museum) and Leslie Chisholm (South Australian Museum) for their curatorial assistance throughout this study. Numerous friends, family and colleagues assisted in the collection of assassin spiders in the field, including Greta Binford, Sarah Comer, Alan Danks, Volker Framenau, Danilo Harms, Stephanie Harms, Lara Lopardo, Melinda Moir, Janet Newell, Ricardo Ott, Dale Roberts, Beverley Roberts, Roy Teale, Janine Wojcieszek and Hannah Wood. Thanks also to Nicholas Porch and the Deakin University Wildlife Field Studies students for first discovering archaeids in the Otway Range and making the holotype specimen of Zephyrarchaea porchi available to MGR prior to the completion of this revision. Specimens were collected under permit throughout Victoria (Permit No. 10005193), South Australia (Permit No. W25803) and Western Australia (Permit Nos. SF005357, SF005814, SF006247, SF006821). Finally, thanks to Hannah Wood and Charles Griswold for providing data on Australian specimens housed at the California Academy of Sciences (CAS), and for assisting MGR and MSH during their visit to the CAS in 2011.
Habitus images of live Archaeidae from southern Australia: A paratype female Zephyrarchaea vichickmani sp. n. from Acheron Gap, Central Highlands, Victoria; B holotype male Zephyrarchaea barrettae sp. n. from Talyuberlup Peak, Stirling Range National Park, Western Australia; C paratype female Zephyrarchaea janineae sp. n. from Karri Valley, Western Australia; D male Zephyrarchaea marki sp. n. from Thistle Cove, Cape Le Grand National Park, Western Australia; E female Zephyrarchaea mainae (Platnick) from William Bay National Park, Western Australia; F female Zephyrarchaea mainae from Bremer Bay, Western Australia.
Habitus images of live Archaeidae from southern Australia: A paratype female Zephyrarchaea vichickmani sp. n. from Acheron Gap, Central Highlands, Victoria; B holotype male Zephyrarchaea barrettae sp. n. from Talyuberlup Peak, Stirling Range National Park, Western Australia; C paratype female Zephyrarchaea janineae sp. n. from Karri Valley, Western Australia; D male Zephyrarchaea marki sp. n. from Thistle Cove, Cape Le Grand National Park, Western Australia; E female Zephyrarchaea mainae (Platnick) from William Bay National Park, Western Australia; F female Zephyrarchaea mainae from Bremer Bay, Western Australia.
Map showing the known distribution of Archaeidae in Australia, with locality records for species of Zephyrarchaea highlighted in black. Note the disjunct distribution of the genus in south-eastern and south-western mainland Australia.
Map showing the known distribution of Archaeidae in Australia, with locality records for species of Zephyrarchaea highlighted in black. Note the disjunct distribution of the genus in south-eastern and south-western mainland Australia.
Phylogeny of Zephyrarchaea species from Rix and Harvey (2012), showing results from a combined, gene-partitioned Bayesian analysis of that study’s multi-gene dataset (2591 bp: COI, COII, tRNA-K, tRNA-D, ATP8, ATP6, H3). Clades with >95% posterior probability support are denoted by thickened branches, with lower individual clade support values shown above nodes. Note the presence of three main regional clades, each illustrated with an exemplar species (highlighted*). The Victorian species Zephyrarchaea grayi sp. n. and Zephyrarchaea porchi sp. n. were not able to be sequenced for this study (shown as black dots on inset map).
Phylogeny of Zephyrarchaea species from Rix and Harvey (2012), showing results from a combined, gene-partitioned Bayesian analysis of that study’s multi-gene dataset (2591 bp: COI, COII, tRNA-K, tRNA-D, ATP8, ATP6, H3). Clades with >95% posterior probability support are denoted by thickened branches, with lower individual clade support values shown above nodes. Note the presence of three main regional clades, each illustrated with an exemplar species (highlighted*). The Victorian species Zephyrarchaea grayi sp. n. and Zephyrarchaea porchi sp. n. were not able to be sequenced for this study (shown as black dots on inset map).
Diagnostic characters of Zephyrarchaea gen. n. and Austrarchaea Forster & Platnick. A–B, Cephalothorax, lateral view, showing differences in carapace height and the position of accessory setae on male chelicerae: A, male Austrarchaea harmsi Rix & Harvey; B, male Zephyrarchaea marki sp. n. C–D, Expanded male pedipalps, retro-ventral view, showing differences in the articulation and fusion of the conductor sclerites: C, Austrarchaea helenae Rix & Harvey; D, Zephyrarchaea marae sp. n. bH = basal haematodocha; C = conductor; C1–2 = conductor sclerites 1–2; Cy = cymbium; E = embolus; Es = embolic sclerite; H = distal haematodocha; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: C–D = 0.2 mm.<br/>
Diagnostic characters of Zephyrarchaea gen. n. and Austrarchaea Forster & Platnick. A–B, Cephalothorax, lateral view, showing differences in carapace height and the position of accessory setae on male chelicerae: A, male Austrarchaea harmsi Rix & Harvey; B, male Zephyrarchaea marki sp. n. C–D, Expanded male pedipalps, retro-ventral view, showing differences in the articulation and fusion of the conductor sclerites: C, Austrarchaea helenae Rix & Harvey; D, Zephyrarchaea marae sp. n. bH = basal haematodocha; C = conductor; C1–2 = conductor sclerites 1–2; Cy = cymbium; E = embolus; Es = embolic sclerite; H = distal haematodocha; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: C–D = 0.2 mm.<br/>
Carapace morphology of Zephyrarchaea species. A–B, Zephyrarchaea mainae (Platnick): A, male pars cephalica, dorso-lateral view, showing accessory setae (AS) on and adjacent to proximal cheliceral bulge; B, female pars cephalica, antero-lateral view, showing cheliceral foramen (CF) and ocular bulge (OB). C–D, Zephyrarchaea marae sp. n.: C, male cephalothorax, antero-lateral view; D, male pars cephalica and chelicerae, frontal view, showing dorsal ‘head’ region and cheliceral foramen (CF). E, Cheliceraeof male Zephyrarchaea mainae, lateral view, showing proximal accessory setae (AS) and ectal stridulatory file (SF). F–G, Detail of carapace of male Zephyrarchaea marae, lateral view, showing granulate cuticle and setose tubercles (sT).<br/>
Carapace morphology of Zephyrarchaea species. A–B, Zephyrarchaea mainae (Platnick): A, male pars cephalica, dorso-lateral view, showing accessory setae (AS) on and adjacent to proximal cheliceral bulge; B, female pars cephalica, antero-lateral view, showing cheliceral foramen (CF) and ocular bulge (OB). C–D, Zephyrarchaea marae sp. n.: C, male cephalothorax, antero-lateral view; D, male pars cephalica and chelicerae, frontal view, showing dorsal ‘head’ region and cheliceral foramen (CF). E, Cheliceraeof male Zephyrarchaea mainae, lateral view, showing proximal accessory setae (AS) and ectal stridulatory file (SF). F–G, Detail of carapace of male Zephyrarchaea marae, lateral view, showing granulate cuticle and setose tubercles (sT).<br/>
Abdominal morphology of Zephyrarchaea species. A–B, Male abdomens, dorso-lateral view, showing dorsal scutes (S) and additional dorsal sclerites (ds) on hump-like tubercles: A, Zephyrarchaea mainae (Platnick); B, Zephyrarchaea marae sp. n. C, Female epigastric region of Zephyrarchaea mainae, ventral view, showing setose book lung covers (BL) and median genital plate (GP). D, Male epigastric region of Zephyrarchaea mainae, ventral view, showing fusion of epigastric sclerites. E, Abdominal cuticle of male Zephyrarchaea marae, lateral view, showing sclerotic spots (ss) surrounded by short setae. F, Spinnerets of female Zephyrarchaea mainae, posterior view (ventral side uppermost), showing anterior lateral (ALS) and posterior lateral (PLS) spinnerets anterior to anal tubercle (AT), and the absence of a colulus.
Abdominal morphology of Zephyrarchaea species. A–B, Male abdomens, dorso-lateral view, showing dorsal scutes (S) and additional dorsal sclerites (ds) on hump-like tubercles: A, Zephyrarchaea mainae (Platnick); B, Zephyrarchaea marae sp. n. C, Female epigastric region of Zephyrarchaea mainae, ventral view, showing setose book lung covers (BL) and median genital plate (GP). D, Male epigastric region of Zephyrarchaea mainae, ventral view, showing fusion of epigastric sclerites. E, Abdominal cuticle of male Zephyrarchaea marae, lateral view, showing sclerotic spots (ss) surrounded by short setae. F, Spinnerets of female Zephyrarchaea mainae, posterior view (ventral side uppermost), showing anterior lateral (ALS) and posterior lateral (PLS) spinnerets anterior to anal tubercle (AT), and the absence of a colulus.
Graphs depicting the relationship between carapace length (CL) and carapace height (CH) for species of Zephyrarchaea. Smaller grey dots denote species of Austrarchaea (see Rix and Harvey 2011); boxes denote the three lineages of Zephyrarchaea from Victoria/South Australia, the Stirling Range and elsewhere in southern Western Australia.<br/>
Graphs depicting the relationship between carapace length (CL) and carapace height (CH) for species of Zephyrarchaea. Smaller grey dots denote species of Austrarchaea (see Rix and Harvey 2011); boxes denote the three lineages of Zephyrarchaea from Victoria/South Australia, the Stirling Range and elsewhere in southern Western Australia.<br/>
Lateral ‘head’ profiles of males of species of Zephyrarchaea, showing variation in carapace shape as quantified by the post-ocular ratio (P.O. Ratio) and ratio of highest point of carapace relative to post-ocular length (HPC Ratio): A, holotype Zephyrarchaea marae sp. n., showing the derivation of morphometric ratios; B, holotype Zephyrarchaea vichickmani sp. n.; C, holotype Zephyrarchaea marae sp. n.; D, holotype Zephyrarchaea porchi sp. n.; E, holotype Zephyrarchaea melindae sp. n.; F, holotype Zephyrarchaea barrettae sp. n.; G, holotype Zephyrarchaea mainae (Platnick, 1991b); H, holotype Zephyrarchaea marki sp. n.; I, holotype Zephyrarchaea janineae sp. n.<br/>
Lateral ‘head’ profiles of males of species of Zephyrarchaea, showing variation in carapace shape as quantified by the post-ocular ratio (P.O. Ratio) and ratio of highest point of carapace relative to post-ocular length (HPC Ratio): A, holotype Zephyrarchaea marae sp. n., showing the derivation of morphometric ratios; B, holotype Zephyrarchaea vichickmani sp. n.; C, holotype Zephyrarchaea marae sp. n.; D, holotype Zephyrarchaea porchi sp. n.; E, holotype Zephyrarchaea melindae sp. n.; F, holotype Zephyrarchaea barrettae sp. n.; G, holotype Zephyrarchaea mainae (Platnick, 1991b); H, holotype Zephyrarchaea marki sp. n.; I, holotype Zephyrarchaea janineae sp. n.<br/>
Lateral ‘head’ profiles of females of species of Zephyrarchaea, showing variation in carapace shape as quantified by the post-ocular ratio (P.O. Ratio) and ratio of highest point of carapace relative to post-ocular length (HPC Ratio) (see Fig. 8): A, allotype Zephyrarchaea vichickmani sp. n.; B, allotype Zephyrarchaea marae sp. n.; C, holotype Zephyrarchaea grayi sp. n.; D, holotype Zephyrarchaea austini sp. n.; E, Zephyrarchaea mainae (Platnick, 1991b); F, allotype Zephyrarchaea janineae sp. n.; G, holotype Zephyrarchaea robinsi (Harvey, 2002a); H, allotype Zephyrarchaea melindae sp. n.; I, allotype Zephyrarchaea barrettae sp. n.
Lateral ‘head’ profiles of females of species of Zephyrarchaea, showing variation in carapace shape as quantified by the post-ocular ratio (P.O. Ratio) and ratio of highest point of carapace relative to post-ocular length (HPC Ratio) (see Fig. 8): A, allotype Zephyrarchaea vichickmani sp. n.; B, allotype Zephyrarchaea marae sp. n.; C, holotype Zephyrarchaea grayi sp. n.; D, holotype Zephyrarchaea austini sp. n.; E, Zephyrarchaea mainae (Platnick, 1991b); F, allotype Zephyrarchaea janineae sp. n.; G, holotype Zephyrarchaea robinsi (Harvey, 2002a); H, allotype Zephyrarchaea melindae sp. n.; I, allotype Zephyrarchaea barrettae sp. n.
Zephyrarchaea mainae (Platnick, 1991b). A–B, Cephalothorax and abdomen, lateral view: A, female (WAM T118983) from Mutton Bird Point, Western Australia; B, holotype male (WAM T17683) from Torndirrup National Park, Western Australia. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Male (WAM T89569) pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prolateral view. G, Female (WAM T118983) internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea mainae (Platnick, 1991b). A–B, Cephalothorax and abdomen, lateral view: A, female (WAM T118983) from Mutton Bird Point, Western Australia; B, holotype male (WAM T17683) from Torndirrup National Park, Western Australia. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Male (WAM T89569) pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prolateral view. G, Female (WAM T118983) internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea janineae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (WAM T118981) from Karri Valley, Western Australia; B, holotype male (WAM T89559) from Karri Valley, Western Australia. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Zephyrarchaea janineae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (WAM T118981) from Karri Valley, Western Australia; B, holotype male (WAM T89559) from Karri Valley, Western Australia. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.
Zephyrarchaea marki sp. n. A–E, Holotype male (WAM T118985) from Thistle Cove, Cape Le Grand National Park, Western Australia: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb, retrolateral view; E, detail of distal tegular sclerites, prolateral view. C1–2 = conductor sclerites 1–2; E = embolus; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A = 1.0 mm; D = 0.2 mm.<br/>
Zephyrarchaea marki sp. n. A–E, Holotype male (WAM T118985) from Thistle Cove, Cape Le Grand National Park, Western Australia: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb, retrolateral view; E, detail of distal tegular sclerites, prolateral view. C1–2 = conductor sclerites 1–2; E = embolus; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A = 1.0 mm; D = 0.2 mm.<br/>
Zephyrarchaea robinsi (Harvey, 2002a). A–D, Holotype female (WAM T42580) from Ellen Peak, Stirling Range National Park, Western Australia: A, cephalothorax and abdomen, lateral view; B–D, internal genitalia, dorsal view; C–D, illustrations (from Harvey 2002a) of internal genitalia, dorsal view, showing shape of membranous bursa and underlying spermathecae. B = bursa; GP = genital plate; Sp = spermathecae. Scale bar: A = 1.0 mm.<br/>
Zephyrarchaea robinsi (Harvey, 2002a). A–D, Holotype female (WAM T42580) from Ellen Peak, Stirling Range National Park, Western Australia: A, cephalothorax and abdomen, lateral view; B–D, internal genitalia, dorsal view; C–D, illustrations (from Harvey 2002a) of internal genitalia, dorsal view, showing shape of membranous bursa and underlying spermathecae. B = bursa; GP = genital plate; Sp = spermathecae. Scale bar: A = 1.0 mm.<br/>
Zephyrarchaea melindae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (WAM T97468) from Toolbrunup Peak, Stirling Range National Park, Western Australia; B, holotype male (WAM T118986) from Mount Hassell, Stirling Range National Park, Western Australia. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view, with inset showing twisted apex of tegular sclerite 1 in retroventral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1 = tegular sclerite 1. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea melindae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (WAM T97468) from Toolbrunup Peak, Stirling Range National Park, Western Australia; B, holotype male (WAM T118986) from Mount Hassell, Stirling Range National Park, Western Australia. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view, with inset showing twisted apex of tegular sclerite 1 in retroventral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1 = tegular sclerite 1. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea barrettae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (WAM T97466) from Talyuberlup Peak, Stirling Range National Park, Western Australia; B, holotype male (WAM T117055) from Talyuberlup Peak, Stirling Range National Park, Western Australia. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view, with inset showing twisted apex of tegular sclerite 1 in retroventral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; H = distal haematodocha; T = tegulum; (TS)1 = tegular sclerite 1. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea barrettae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (WAM T97466) from Talyuberlup Peak, Stirling Range National Park, Western Australia; B, holotype male (WAM T117055) from Talyuberlup Peak, Stirling Range National Park, Western Australia. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view, with inset showing twisted apex of tegular sclerite 1 in retroventral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; H = distal haematodocha; T = tegulum; (TS)1 = tegular sclerite 1. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea vichickmani sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (MV K11579) from Acheron Gap, Victoria; B, holotype male (MV K11578) from Acheron Gap, Victoria. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view, with inset showing twisted apex of tegular sclerite 1 in retroventral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea vichickmani sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (MV K11579) from Acheron Gap, Victoria; B, holotype male (MV K11578) from Acheron Gap, Victoria. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view, with inset showing twisted apex of tegular sclerite 1 in retroventral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea marae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (MV K5921) from Gunyah Rainforest State Reserve, Victoria; B, holotype male (MV K11580) from Tarra-Bulga National Park, Victoria. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view, with inset showing twisted apex of tegular sclerite 1 in retroventral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea marae sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (MV K5921) from Gunyah Rainforest State Reserve, Victoria; B, holotype male (MV K11580) from Tarra-Bulga National Park, Victoria. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, retrolateral view, with inset showing twisted apex of tegular sclerite 1 in retroventral view; F, detail of distal tegular sclerites, prolateral view. G, Allotype female internal genitalia, dorsal view. C1–2 = conductor sclerites 1–2; E = embolus; GP = genital plate; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A–B = 1.0 mm; E = 0.2 mm.<br/>
Zephyrarchaea porchi sp. n. A–E, Holotype male (MV K11581) from Bimbi Park, Otway Range, Victoria: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb (partially expanded), retrolateral view; E, detail of distal tegular sclerites, prolateral view. C1–2 = conductor sclerites 1–2; E = embolus; eH = embolic (distal) haematodocha; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Zephyrarchaea porchi sp. n. A–E, Holotype male (MV K11581) from Bimbi Park, Otway Range, Victoria: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb (partially expanded), retrolateral view; E, detail of distal tegular sclerites, prolateral view. C1–2 = conductor sclerites 1–2; E = embolus; eH = embolic (distal) haematodocha; T = tegulum; (TS)1–3 = tegular sclerites 1–3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Zephyrarchaea grayi sp. n. and Zephyrarchaea austini sp. n. A–B, holotype female Zephyrarchaea grayi sp. n. (AMS KS109448) from Delley’s Dell, Grampians National Park, Victoria: A, cephalothorax and abdomen, lateral view; B, internal genitalia, antero-dorsal view. C–D, holotype female Zephyrarchaea austini (SAM NN28000) from Western River Wilderness Protection Area, Kangaroo Island, South Australia: C, cephalothorax and abdomen, lateral view; D, internal genitalia, antero-dorsal view. GP = genital plate. Scale bars: A, C = 1.0 mm.
Zephyrarchaea grayi sp. n. and Zephyrarchaea austini sp. n. A–B, holotype female Zephyrarchaea grayi sp. n. (AMS KS109448) from Delley’s Dell, Grampians National Park, Victoria: A, cephalothorax and abdomen, lateral view; B, internal genitalia, antero-dorsal view. C–D, holotype female Zephyrarchaea austini (SAM NN28000) from Western River Wilderness Protection Area, Kangaroo Island, South Australia: C, cephalothorax and abdomen, lateral view; D, internal genitalia, antero-dorsal view. GP = genital plate. Scale bars: A, C = 1.0 mm.
Zephyrarchaea mainae (Platnick, 1991b), distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea mainae highlighted in yellow (red highlighted localities denote juvenile specimens of tentative identification; see Zephyrarchaea sp. unidentified juvenile specimens, above); B, satellite image showing detail of inset (A); C, temperate coastal heathland near the type locality – Albany Wind Farm, Torndirrup Peninsula, Western Australia (March 2008). Image (C) by M. Rix.
Zephyrarchaea mainae (Platnick, 1991b), distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea mainae highlighted in yellow (red highlighted localities denote juvenile specimens of tentative identification; see Zephyrarchaea sp. unidentified juvenile specimens, above); B, satellite image showing detail of inset (A); C, temperate coastal heathland near the type locality – Albany Wind Farm, Torndirrup Peninsula, Western Australia (March 2008). Image (C) by M. Rix.
Zephyrarchaea janineae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea janineae highlighted in yellow; B, satellite image showing detail of inset (A); C, wet eucalypt forest at the type locality – Karri Valley, Western Australia (August 2006). Image (C) by M. Rix.
Zephyrarchaea janineae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea janineae highlighted in yellow; B, satellite image showing detail of inset (A); C, wet eucalypt forest at the type locality – Karri Valley, Western Australia (August 2006). Image (C) by M. Rix.
Zephyrarchaea marki sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea marki highlighted in yellow; B, satellite image showing detail of inset (A); C, temperate coastal heathland at the type locality – Thistle Cove, Cape Le Grand National Park, Western Australia (June 2010). Image (C) by M. Rix.
Zephyrarchaea marki sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea marki highlighted in yellow; B, satellite image showing detail of inset (A); C, temperate coastal heathland at the type locality – Thistle Cove, Cape Le Grand National Park, Western Australia (June 2010). Image (C) by M. Rix.
Zephyrarchaea robinsi (Harvey, 2002a), distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea robinsi highlighted in yellow; B, satellite image showing detail of inset (A); C, montane heathland at the type locality – Ellen Peak, Stirling Range National Park, Western Australia (November 2007), with Bluff Knoll visible in the distance. Image (C) by M. Rix.
Zephyrarchaea robinsi (Harvey, 2002a), distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea robinsi highlighted in yellow; B, satellite image showing detail of inset (A); C, montane heathland at the type locality – Ellen Peak, Stirling Range National Park, Western Australia (November 2007), with Bluff Knoll visible in the distance. Image (C) by M. Rix.
Zephyrarchaea melindae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea melindae highlighted in yellow; B, satellite image showing detail of inset (A); C, view from the summit of Bluff Knoll across the western Stirling Range National Park, showing collection localities for Zephyrarchaea melindae (i.e. Toolbrunup Peak, Mount Hassell) highlighted in bold (June 2010). Note the Chester Pass lowlands, separating populations of Zephyrarchaea melindae and Zephyrarchaea robinsi, and Talyuberlup Peak in the distance, home to Zephyrarchaea barrettae sp. n. Image (C) by M. Rix.
Zephyrarchaea melindae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea melindae highlighted in yellow; B, satellite image showing detail of inset (A); C, view from the summit of Bluff Knoll across the western Stirling Range National Park, showing collection localities for Zephyrarchaea melindae (i.e. Toolbrunup Peak, Mount Hassell) highlighted in bold (June 2010). Note the Chester Pass lowlands, separating populations of Zephyrarchaea melindae and Zephyrarchaea robinsi, and Talyuberlup Peak in the distance, home to Zephyrarchaea barrettae sp. n. Image (C) by M. Rix.
Zephyrarchaea barrettae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea barrettae highlighted in yellow; B, satellite image showing detail of inset (A); C, view from Stirling Range Drive showing the type locality – Talyuberlup Peak, Stirling Range National Park (August 2008). Image (C) by M. Rix.
Zephyrarchaea barrettae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in south-western Western Australia, with collection localities for Zephyrarchaea barrettae highlighted in yellow; B, satellite image showing detail of inset (A); C, view from Stirling Range Drive showing the type locality – Talyuberlup Peak, Stirling Range National Park (August 2008). Image (C) by M. Rix.
Zephyrarchaea vichickmani sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea vichickmani highlighted in yellow; B, satellite image showing detail of inset (A); C, cool-temperate Nothofagus rainforest at the type locality – Acheron Gap, Yarra Ranges National Park, Victoria (March 2010). Image (C) by M. Rix.
Zephyrarchaea vichickmani sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea vichickmani highlighted in yellow; B, satellite image showing detail of inset (A); C, cool-temperate Nothofagus rainforest at the type locality – Acheron Gap, Yarra Ranges National Park, Victoria (March 2010). Image (C) by M. Rix.
Zephyrarchaea marae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea marae highlighted in yellow; B, satellite image showing detail of inset (A); C, cool-temperate Nothofagus rainforest at the type locality – Tarra Valley, Tarra-Bulga National Park, Victoria (April 2010). Image (C) by M. Rix.
Zephyrarchaea marae sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea marae highlighted in yellow; B, satellite image showing detail of inset (A); C, cool-temperate Nothofagus rainforest at the type locality – Tarra Valley, Tarra-Bulga National Park, Victoria (April 2010). Image (C) by M. Rix.
Zephyrarchaea porchi sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea porchi highlighted in yellow; B, satellite image showing detail of inset (A); C, bracken-rich eucalypt forest at the type locality – Bimbi Park, Otway Range, Victoria (March 2012). Image (C) by N. Porch, used with permission.
Zephyrarchaea porchi sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea porchi highlighted in yellow; B, satellite image showing detail of inset (A); C, bracken-rich eucalypt forest at the type locality – Bimbi Park, Otway Range, Victoria (March 2012). Image (C) by N. Porch, used with permission.
Zephyrarchaea grayi sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea grayi highlighted in yellow; B, satellite image showing detail of inset (A); C, wet sclerophyll forest at the type locality – Delley’s Dell, Grampians National Park, Victoria (March 2010). Image (C) by M. Rix.
Zephyrarchaea grayi sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea grayi highlighted in yellow; B, satellite image showing detail of inset (A); C, wet sclerophyll forest at the type locality – Delley’s Dell, Grampians National Park, Victoria (March 2010). Image (C) by M. Rix.
Zephyrarchaea austini sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea austini highlighted in yellow; B, satellite image showing detail of inset (A); C, open eucalypt woodland and heathland at the type locality – near Billy Goat Falls, Western River Wilderness Protection Area, Kangaroo Island, South Australia (May 2010). Image (C) by M. Rix.
Zephyrarchaea austini sp. n., distribution and habitat: A, topographic map showing the known distribution of Archaeidae in Victoria and South Australia, with collection localities for Zephyrarchaea austini highlighted in yellow; B, satellite image showing detail of inset (A); C, open eucalypt woodland and heathland at the type locality – near Billy Goat Falls, Western River Wilderness Protection Area, Kangaroo Island, South Australia (May 2010). Image (C) by M. Rix.